Immunoglobulins G, of which there are four subclasses (IgG1, IgG2, IgG3 and IgG4) are the quantitatively class of antibody more represented in the serum, but are also present in the extravascular environement.
They are produced during the secondary response and activate the classical way of complement (interaction of C1, C4 and C2 with the antigen-antibody complex). IgGs play a role during the opsonisation of microorganisms encouraging their phagocytosis by specific receptors (FcgRI) for the Fc fragment of IgG expressed on the cell membranes of neutrophils and macrophages.

IgG function

The binding of IgG on the surface of foreign cells increases the efficiency of cell lysis (ADCC mechanism: antibody-dependent cell-mediated cytotoxicity) by NK cells, using a low-affinity receptor expressed by these cells: the FcgRIII.
Other low-affinity receptors (FcgRII) are expressed by monocytes, macrophages, neutrophils, eosinophils, platelets and B lymphocytes. These receptors facilitate the removal of circulating immune complexes from cells engulf.
Fc-gR : receptor for Fc fragment of IgG
FcgR belong to the superfamily of immunoglobulins and plays a vital role in the phagocytosis of opsonised microorganisms.
The family of Fc-gR includes many subtypes, such as FcγRI (CD64), FcγRIIA (CD32), FcγRIIB (CD32), FcγRIIIA (CD16a) and FcgRIIIB (CD16b), each of which differs from the others by a different affinity towards antibodies, inequality due to a different molecular structure.
FcγRI binds more strongly than IgG they do FcgRII and FcgRIII. This increased affinity is due to an extracellular portion composed of three Ig-like domains, one in more than FcgRII and FcgRIII. This property allows the activation of FcgRI by one molecule of IgG without which an immune complex can not be activated.
This class of receptors has other features: the structure is similar to that of MHC class I and is involved in antibody transfer from mother to fetus during pregnancy and after during lactation (FcRn: neonatal Fc receptor).
Fc-gR modulation by Genistein

Kawasaki disease and soy: potential role for isoflavone interaction with Fcγ receptors, 2013
Kawasaki disease is a diffuse vasculitis occurring in children and showing predilection for the coronary arteries. Differences in diet between Asians and Westerners are touted as reasons for certain ethnic-related discrepancies in susceptibility to cardiovascular disease and cancer in adults. Surprisingly, these cultural dietary differences have not been previously considered as the source of the discrepancy in KD incidence among these ethnic populations. Recent data from genetic studies have highlighted the role of specific immune receptors in the pathogenesis of KD. Functions of the Fcγ receptors (FcGRs) are modulated by isoflavones in soy, in particular, genistein. Epidemiological data from Hawaiian populations support an association between soy consumption and KD. These observations form the basis of a hypothesis: isoflavones participate in KD pathogenesis by modulating function of the FcGRs and by disrupting the balance between activation and inhibition of the inflammatory response.
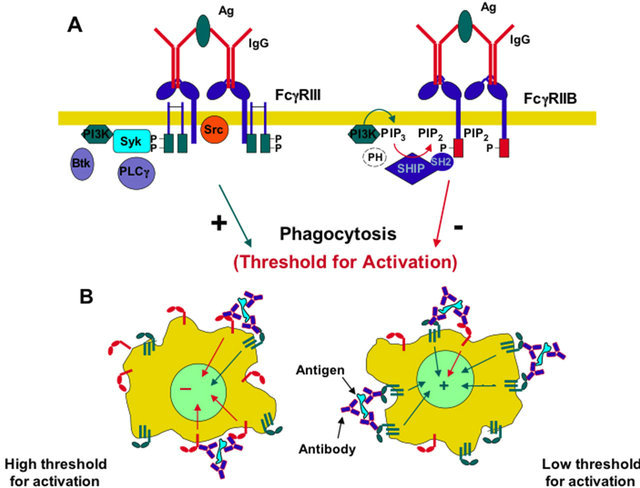
Fc receptors: Cell activators of antibody functions, 2013
IgG molecules bind to their cognate antigens and are in turn recognized by specific receptors (Fcγ receptors) on the membrane of leukocytes. Crosslinking these receptors on the surface of leukocytes leads to activation of several effector cell functions. These effector functions are geared toward the destruction of microbial pathogens and the induction of an inflammatory state that is beneficial during infections. However, in autoimmune diseases, antibodies can direct these effector functions against normal tissues and cause severe tissue damage. In recent years, several factors that can modulate the IgG-FcγR interaction have been elucidated. In this review, we describe the main types of Fcγ receptors, and our current view of how antibody variants interact with these receptors to initiate different cell responses.

Mechanism of degradation of monoclonal antibodies. FcgR, Fc gamma receptor; FcRn, Brambell receptor.
FcgR generate signals through an activator site called
ITAM (Immunoreceptor tyrosine-based activation motif). Each
ITAM is composed by a pattern of amino acids (YXXL) in succession in the queue dual intracellular receptor. When thyroxines (Y) are added to the phosphate groups by action of specific tyrosine kinases, the signal is transmitted within the cell. This phosphorylation always coupling the Fc receptor with its specific ligand.
ITAM stimulates phagocytosis in macrophages. FcgRI and FcgRIIIA lack of
ITAM but still transmit the signal using another protein function. This molecule, called
FCG subunit contains two patterns (YXXL) characteristic of the
ITAM, which, in effect, override the role played by this molecule.
These two patterns must be present in two copies, because one of the two (ITIM) seems to have inhibitory effects against phagocytosis. The inhibition is regulated by two enzymes,
SHP-1 and
SHP-2 that removes phosphate groups from tyrosine residues, thus blocking the positive response to the stimulus (Figure 2).

FCR recognizes microorganisms that have been linked by Abs (Figure 3). The interaction between antibodies bound to the surface of the cell and
FCR actives cell of the immune system to destroy the target.

Figure 3: The picture depicts the action of macrophages against a microorganism coated with antibodies"
IgG synthesis
Signaling Proteins and Transcription Factors in Normal and Malignant Early B Cell Development, 2011

Figure 1: B cell development in adult mammals starts in bone marrow with the commitment of hematopoietic stem cells (HSCs) to the B cell lineage and ends with formation of mature B cells in peripheral secondary lymphoid organs (e.g., the spleen). It is the sequential expression and assembly of the components of the B cell antigen receptor (BCR) what defines each developmental stage. The first stage exhibiting commitment to the B cell lineage is the proB, and here the immunoglobulin heavy chain is in the process of recombination, and the signaling proteins Igα and Igβ are in surface forming complexes with chaperon proteins like calnexin (the proBCR). The next developmental stage, the preB, happens after the heavy chain was successfully recombined and the preBCR is assembled. In this stage, the light chain is recombined and unrearranged heavy chain alleles are excluded. After light chain recombination and pairing with the heavy chain and Igα and Igβ the mature BCR is formed, the B cell is in the immature (in bone marrow) and transitional (in periphery) stages. Here, B cell mechanisms of self-tolerance are active allowing self- and nonself-recognition by the mature B cell. Transition to the mature stage happens if the BCR of the immature/transitional B cell does not find its cognate antigen after several days of bone marrow and peripheral trafficking.

Figure 2: (a) The Ig heavy and light chain genes are comprised of constant and variable regions, where the variable region is formed by an n number of segments termed V (variable), D (diversity), and J (joining) in the heavy chain and by segments V and J in the light chain. These segments are brought together by a site-specific recombination process termed VDJ recombination responsible for the extensive repertoire of BCR specificities. There are two loci for light chain, κ and λ. Here, all the loci are shown in germline configuration, previous to the process of VDJ recombination. (b) The early stages of B cell development are differentiated by the process of VDJ recombination, and the heavy (IgH) and light (IgL) chains are recombined in the proB and preB stages, respectively. Each stage is further subdivided according to the sequential assembly of the VDJ segments. Replication and recombination processes are mutually exclusive as denoted by the circular arrows and VDJ signs inside the cell. Dashed lines separating proB and preB stages indicate checkpoints where signaling from the preBCR and BCR is required for positive selection and progression along the B cell maturation pathway. Continuous lines indicate the main receptors controlling each developmental stage. The differential intensity in the IL-7 green line indicates the sub-stages where a higher or lower concentration of the IL-7R ligand is required.

Figure 3: Homeostatic and leukemic expression of receptors, signaling proteins, and transcription factors along the B cell pathway. Developmental stages are indicated starting with the hematopoietic stem cell (HSC), the common lymphoid progenitor (CLP), and into the B cell pathway, stages proB, preB, and mature B cells. Normal gene expression along the developmental pathway is indicated with blue bars, and expression dependency between proteins is indicated with arrows. Most common modified forms of these receptors, signaling proteins, and transcription factors associated with acute lymphoblastic leukemia are also indicated.
Free immunoglobulin light chain: Its biology and implications in diseases 2011 Fulltext
additional links
Molecular features responsible for the absence of immunoglobulin heavy chain protein synthesis in an IgH− subgroup of multiple myeloma 2000
Synthesis and secretion of light-chain immunoglobulin in two successive cycles of synchronized plasmacytoma cells, 1976
Biology of immunoglobulin light chains

Immunohistochemical staining of κ producing bone marrow plasma cells from a patient with MM using fluorescein-conjugated, anti-κ antiserum. 5 plasma cells can be seen. B. Diagrammatic representation of plasma cells producing intact immunoglobulins with monomeric κ and dimeric λ FLC molecules.
Plasma cells produce one of five heavy chain types, together with κ or λ molecules. There is approximately 40% excess FLC production over heavy chain synthesis, to allow proper conformation of the intact immunoglobulin molecules. As mentioned previously, there are twice as many κ-producing plasma cells as λ-producing plasma cells. κ FLCs are normally monomeric, while λ FLCs tend to be dimeric, joined by disulphide bonds, however higher polymeric forms of both FLCs may occur

Nephron showing filtration, metabolism and excretion of FLCs.
Because of the huge metabolic capacity of the proximal tubule, the amount of FLCs in urine, even when production is considerably increased, is more dependent upon renal function than synthesis by the tumour. As a consequence, serum and urine FLC concentrations may not be similar during the evolution of LCMM. This is shown in a hypothetical patient in Figure 3.8. The red line shows the steady increase in sFLCs as the tumour grows over the first 12 months. When synthesis of FLCs exceeds 10-30g/day (greater than 30 times normal) there is an overflow proteinuria and large amounts of FLCs enter the urine. It is normally at this point that patients with LCMM are identified.
Co-translational modification of nascent immunoglobulin heavy and light chains., 1979
The nucleotide sequence of a methionine tRNA which functions in protein elongation in mouse myeloma cells. 1975
IgG synthesis regulation
Liver-X-receptor activator prevents homocysteine-induced production of IgG antibodies from murine B lymphocytes via the ROS-NF-kappaB pathway. 2007
- Our previous study showed that homocysteine (Hcy) promotes proliferation of mouse splenic B lymphocytes. In this study, we investigated whether Hcy could stimulate the production of IgG antibodies. Hcy significantly increased the production of IgG antibodies from resting B lymphocytes. B lymphocytes from ApoE-knockout mice with hyperhomocysteinemia showed elevated IgG secretion at either the basal Hcy level or in response to lipopolysaccharide. Hcy promoted reactive oxygen species (ROS) formation, and free radical scavengers, MnTMPyP decreased Hcy-induced IgG secretion. The inhibitor of NF-kappaB (MG132) also significantly reduced Hcy-induced IgG secretion. Furthermore, Hcy-induced formation of ROS, activation of NF-kappaB, and secretion of IgG could be inhibited by the liver-X-receptor (LXR) agonist T0901317. Thus, our data provide strong evidence that HHcy induces IgG production from murine splenic B lymphocytes both in vitro and in vivo. The mechanism might be through the ROS-NF-kappaB pathway and can be attenuated by the activation of LXR.
Down Syndrome: low Methionine
 down syndrome Igg
down syndrome Igg
Neonates with Downs syndrome but not other chromosomal abnormalities are associated with a deficiency of IgG.
DNA Methylation
Localized DNA Demethylation at Recombination Intermediates during Immunoglobulin Heavy Chain Gene Assembly, 2013
CHAPTER 83 FUNCTIONS OF B LYMPHOCYTES AND PLASMA CELLS IN IMMUNOGLOBULIN PRODUCTION, Williams Hematology
Free immunoglobulin light chain: Its biology and implications in diseases, 2011
- Immunoglobulin light chain (IgLC) is a component of antibodies, but its free form is observed in the circulation, which originates from 10 to 40% excess synthesis over heavy chain in B cells. Complete antibodies function as a defined tetramer structure unit, H2L2; thus, separation of heavy and light chains results in considerable or complete loss of antigen-binding ability. Free IgLC has been considered as an inconsequential spillover during antibody assembly because, unlike heavy chain, neither effector functions such as complement activation nor specific-receptor binding has been identified in IgLCs. Free IgLC in sera and cerebrospinal fluids increases in inflammatory diseases such as autoimmune diseases and infections, presumably as a result of B-cell activation. This may be just a concomitant event during elevated disease activity, but recent findings suggest that free IgLC is involved in a wide range of immunological phenomena as a signaling effector or an anti-inflammatory molecule. These effects are likely to be intrinsic to IgLC. In this review, we attempt to give a comprehensive view about the biological roles of free IgLC together with the gene expression, secretion, antigen-binding ability, and its metabolic characteristics.
Fulltext