DEFINITION
Painful bladder syndrome is the term used to refer to a chronic symptom complex of urinary frequency and bladder ‘pressure’, discomfort or pain in the absence of any other reasonable cause for these symptoms (such as infection).
Interstitial cystitis is the established term used by the National Institute of Diabetes and Digestive and Kidney Diseases consensus workshop for which a research definition was formulated in the late 1980s.
The two terms are often used interchangeably in clinical practice.
Historically, interstitial cystitis (IC) was the name given to the condition, which was associated with severe cystoscopic changes of Hunner’s ulcer, fissuring and reduced bladder capacity.
‘To be diagnosed with interstitial cystitis, patients must have either glomerulations on cystoscopic examination or a classic Hunner’s ulcer, and they must have either pain associated with the bladder or urinary urgency. The presence of any one of the following criteria excludes the diagnosis of interstitial cystitis.
- Bladder capacity of greater than 350 cm3 on awake cystometry using either a gas or liquid filling medium.
- Absence of an intense urge to void with the bladder filled to 100 cm3 of gas or 150 cm3 of water during cystometry, using a fill rate of 30–100 cm3/minute.
- The demonstration of phasic involuntary bladder contractions on cystometry using the fill rate described above.
- Duration of symptoms of less than 9 months.
- Absence of nocturia.
- Symptoms relieved by antimicrobials, urinary antiseptics, anticholinergics, or antispasmodics.
- A frequency of urination, while awake, of less than eight times per day.
- A diagnosis of bacterial cystitis or prostatitis within a 3-month period.
- Bladder or ureteral calculi.
- Active genital herpes.
- Uterine, cervical, vaginal, or urethral cancer.
- Urethral diverticulum.
- Cyclophosphamide or any type of chemical cystitis.
- Tuberculous cystitis.
- Radiation cystitis.
- Benign or malignant bladder tumours.
- Vaginitis.
- Age less than 18 years.

In any case, the term ‘interstitial cystitis’ is a misnomer, with features of inflammation not always present, and there is more evidence for epithelial rather than ‘interstitial’ involvement. However, there is a great reluctance to do away with the term IC because of the public, medical and political awareness of this condition—in no small part due to the work of the IC associations around the world.
The current consensus terminology is that this condition may be referred to as PBS/IC. Painful bladder syndrome has been defined by the International Continence Society as "the complaint of suprapubic pain related to bladder filling, accompanied by other symptoms such as increased daytime and night-time frequency, in the absence of proven urinary infection or other obvious pathology". The ICS uses the term interstitial cystitis for the symptom syndrome associated with typical cystoscopic and histological features.
EPIDEMIOLOGY
The study of the epidemiology of IC/PBS has been confounded by the lack of a uniform definition for IC/PBS. The difference in prevalence estimates can be explained by reliance on differing or variable diagnostic criteria in addition to the possibility of reduced actual incidence or reduced awareness of the condition. As a result, the population prevalence of IC has been estimated at 18 per 100,000 women in 1975 in Finland, 30 per 100,000 in 1987 in the USA, and 865 per 100,000 women in 1994 in the USA.
Curhan et al utilized the Nurses Health Study (NHS) I and II—two large population-based studies of female, mainly white nurses—and found the prevalence of confirmed IC to be of the order of 60 per 100,000. These studies showed that the delay between onset of symptoms and diagnosis was 5–7 years, which means that the prevalence of symptomatic individuals whose disease was not yet confirmed would be far greater. Leppilahti et al administered a symptom questionnaire and calculated the prevalence of probable IC to be 450/100,000.
A consistent finding is the predominance of women with IC. The male-to-female ratio varies between 1:4.5 in the Japanese cohort and 1:9 in the US population survey. In a series of over 500 IC patients, Koziol found the mean age at the time of first symptoms was 42 years, with 30% younger than 30 years at the onset of symptoms.
The natural history of the condition was followed by the Interstitial Cystitis Database (ICDB) and this confirmed no long-term change in overall disease severity over a period of 48 months in a mainly (92%) female cohort.
Family relationships and responsibilities were said to be adversely affected in 70%. The quality of life of women with IC has been said to be worse than women with end-stage renal disease.
SYMPTOMS
- Over half the women described dyspareunia, daily or constant pain, and described the pain as severe or excruciating.
The pain was described as vaginal in 60% and lower abdominal in 60%. Pain was exacerbated by spicy foods, alcoholic, acidic, carbonated or caffeinated beverages in 50%, by sexual intercourse (50%), stress (60%) and exercise (40%).
There is difficulty in describing and quantifying each of the symptoms, which are part of the painful bladder syndrome.
Daytime and night-time frequency can be altered by reduction of fluid intake, and absolute numbers are therefore not as helpful as volume voided, which can be obtained from a 24-hour urinary diary.
In clinical practice, consistently small urine volumes associated with pain or discomfort, rather than incontinence, is a distinguishing feature from bladder overactivity.
Urgency is also a common complaint, by which is meant a constant desire to void, discomfort or pressure. Recently there has been debate about the definition of urgency, with the ICS definition being ‘desire to void for fear of leakage’.Urgency by this
definition is not usual in IC/PBS; rather it is a painful desire to void or pressure which is the commonest symptom. Classically, the pain is associated with bladder filling and is relieved by emptying. However, it is accepted that pain can be felt in the suprapubic area, vagina, urethra, vulva, perineum or rectum. There are no criteria for the location, severity or character of the pain.
DIAGNOSIS
IC/PBS is a diagnosis of exclusion and requires an assessment of symptoms,physical examination, urine microscopy and—if indicated—urine cytology, imaging, usually cystoscopy and sometimes laparoscopy in order to fully differentiate this from other causes of these symptoms. Helpful to the work-up and management of IC are a urinary diary and symptom questionnaire. Optional investigations include urodynamics and bladder biopsy.
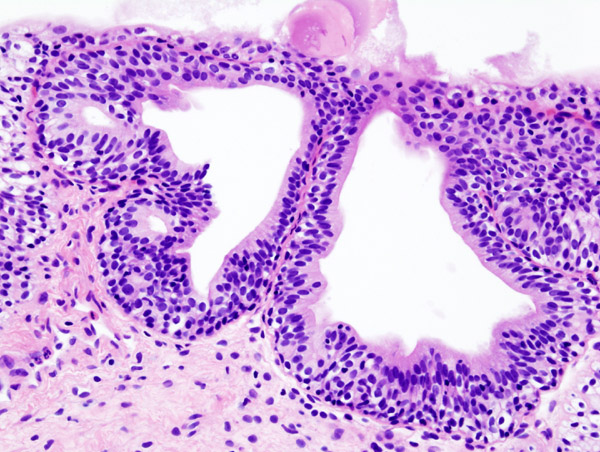
Histopathology
The most commonly reported histological changes in classic IC include:
- epithelial ulceration or denudation
- submucosal inflammation
- granulation tissue formation
- oedema
- congestion
- haemorrhage
- detrusor fibrosis
- increased epithelial, submucosal and detrusor mast cell number
- activation and increased neuronal staining
Early IC is generally characterized by normal or near-normal bladder capacity under anaesthesia and glomerulations. Light microscopy findings range from mucosal ruptures, submucosal haemorrhage and mild inflammation in transurethral resection biopsies to normal histology in forceps biopsies approximately half the time. In other words in the clinical setting of early IC, bladder forceps biopsy pathology may be normal and is not therefore useful as a confirmatory test.
Biopsy pathology can be useful in confirming IC, but usually in the clinical setting of severe disease.
Urine cytology should be mandatory in the population at risk of malignancy, but this would not include women less than 40 years of age. There is also the possibility of false-negative cytology, so that biopsy has an important role if malignancy is suspected.
There are rare occasions when other diagnoses such as eosinophilic cystitis are made. Biopsy remains an optional test in the diagnostic work-up of IC.
Hunner’s ‘‘ulcer’’: is not a chronic ulcer but rather a distinctive inflammatory lesion presenting a characteristic deep rupture through the mucosa and submucosa provoked by bladder distention. The word ‘‘ulcer’’ suggests that it can be seen at cystoscopy without hydrodistention. Consequently, the name Hunner’s ulcer was replaced by Hunner’s lesion. The following definition by Fall was accepted:
‘‘The Hunner’s lesion typically presents as a circumscript, reddened mucosal area with small vessels radiating towards a central scar, with a fibrin deposit or coagulum attached to this area. This site ruptures with increasing bladder distension, with petechial oozing of blood from the lesion and the mucosal margins in a waterfall manner. A rather typical, slightly bullous edema develops post-distension with varying peripheral extension."
Urodynamics
The NIDDK criteria referred to urodynamic assessment in a few ways, but it is not required as part of the work-up of IC/PBS. In the original NIDDK IC patient accrual form, two of a possible four other positive factors were required for a diagnosis of IC apart from Hunner’s ulceration at cystoscopy. One of these factors was decreased bladder compliance.
Furthermore, the revised exclusion criteria included the presence of involuntary bladder contractions, bladder capacity >350 mL or the absence of intense urge to void at 150 mL water. Bladder overactivity was excluded for research purposes in order to clearly demarcate IC patients. However, in the IC database project, 14% had bladder overactivity. Decreased compliance is quite uncommon and has an association with more severe disease.
In practice, urodynamic assessment give limited information in IC/PBS. It is not well
tolerated; usually the only finding is that of reduced awake capacity, which could have been predicted by the urinary diary. It is the overlap with bladder overactivity, which is of interest; however, it is not known whether there is any role for anticholinergic medication in this group.
The cystoscopic findings most often associated with classical descriptions of IC are:
- Hunner’s ulcer, described as patches of red mucosa exhibiting small vessels radiating to a central pale scar or fissure (Figure 1)
- Glomerulations, which are petechial hemorrhages—often with cascade bleeding (Figure 2)—which can be seen during bladder emptying after hydrodistention.
 Figure 1: Hunner’s ulcer in interstitial cystitis: patches of red mucosa exhibiting small vessels radiating to acentral pale scar or fissure.
Figure 1: Hunner’s ulcer in interstitial cystitis: patches of red mucosa exhibiting small vessels radiating to acentral pale scar or fissure.
 Figure 2: Glomerulations in interstitial cystitis.
Figure 2: Glomerulations in interstitial cystitis.
 Figure 3: Interstitial cystitis versus normal bladder.
Figure 3: Interstitial cystitis versus normal bladder.
The cystoscopic capacity may be reduced, in particular in association with ulceration or fissuring. There are many uncertainties about these cystoscopic features.
Glomerulations are most likely a reflection of a chronically underdistended bladder and not specific to IC; not all patients with symptoms of IC/PBS have glomerulations, and not all patients with glomerulations have symptoms of IC/PBS.
There is no clear correlation between cystoscopic appearance and disease severity.
Potassium sensitivity test
The potassium sensitivity test (PST) is an office-based examination in which 40 mL sterile water (control) and 40 mL 0.4N KCl solution (4–10 times physiological urinary potassium concentration) are sequentially instilled into the bladder and the patient is asked to rate the degree of urgency and/or pain (0–5 scale) produced by the two instillations.
- A negative test is when neither water nor potassium provokes any symptoms.
- A positive test is a grading R2 for pain/urgency after potassium compared with water.
The proposed mechanism of action is that the potassium ions are abnormally absorbed through the IC urothelium and stimulate submucosal sensory nerves to produce pain. It has become popular and used as a diagnostic tool particularly in the United States; it has the advantage that it is less costly than cystoscopy, hydrodistension and biopsy.
Biomarkers
The most thoroughly investigated marker for interstitial cystitis is APF, identified and
characterized by Keay and colleagues. They have demonstrated that APF isolated from urine is a heat-stable, low molecular weight, trypsin-sensitive protein that inhibits bladder-cell proliferation and has a sensitivity and specificity >94% for women with documented IC. Furthermore, successful treatment of IC by either hydrodistenstion or neurostimulation normalized APF levels. Keay et al has reported that urine samples in patients with interstitial cystitis contain a factor (antiproliferative factor [APF]) that inhibits bladder epithelial growth. They also found that APF influences changes in specific levels of bladder epithelial growth factors including significantly decreased levels of heparin-binding epidermal growth factor (HB-EGF) and increased levels of epidermal growth factor (EGF) in patients with interstitial cystitis compared to normal controls and patients with other urogenital diseases.
The sensitivity and specificity of APF activity, decreased HB-EGF levels, and increased EGF levels in patients with interstitial cystitis compared with control groups was 94% and 95%, 93% and 89%, and 87% and 91%, respectively. The results are promising, but these assays are currently experimental and are not commercially available.
Questionnaires/quality of life assessment
There are three IC symptom questionnaires:
None of the questionnaires has been shown to be of value in terms of diagnosis.
Radiology
- No known radiographic, ultrasonographic, or other imaging findings are specific for interstitial cystitis.
- Unless indicated to help exclude alternative diagnoses, radiographic studies have only a limited role in the evaluation of interstitial cystitis. Cross-sectional imaging, including MRI, CT scanning, and pelvic ultrasonography, may be performed when clinically indicated to evaluate for a suggestive pelvic mass that is causing compression of the bladder or for an adjacent inflammatory process (eg, diverticulitis).
- Cystography and voiding cystourethrography may be used to evaluate the bladder for other causes of irritative lower urinary tract symptoms, including intravesical masses, stones, bladder diverticula, urethral diverticula, urethral stricture, meatal stenosis, or findings suggestive of a neurogenic or nonneurogenic voiding dysfunction.
Laboratory Tests
No serologic or hematologic abnormalities are known to be specific for interstitial cystitis.
- Various assays for stress protein genes, glycosaminoglycans, mast cell tryptase, Tamm-Horsfall protein autoantibodies, and others have been suggested by numerous investigators; however, these assays are presently used primarily for research purposes and do not have a defined role in the diagnosis of interstitial cystitis.
- In men, expressed prostatic secretions yield no findings specific for interstitial cystitis; nonetheless, localizing cultures and microscopic examination should be performed to exclude bacterial prostatitis.
- Urinalysis and Urine Culture: these tests can detect and identify the most common bacteria in the urine that may be causing interstitial cystitis-like symptoms. A urine sample is obtained either by catheterization or by the "clean catch" method. For a clean catch, the patient washes the genital area before collecting a sample of urine "midstream" in a sterile container. White and red blood cells and bacteria in the urine suggest an infection of the urinary tract that can be treated with antibiotics. If urine is sterile for weeks or months while symptoms persist, a doctor may consider a diagnosis of interstitial cystitis.
- Culture of Prostatic Secretions: this fluid is examined under the microscope for signs of an infection such as red and white blood cells and also can be cultured for bacteria. Prostatic infections can be treated with antibiotics.
PATHOGENESIS
Neurogenic inflammation and mast cells
Neurogenic inflammation has been proposed to be one of the important pathophysiological processes occurring in IC, and includes:
- sensory nerve fibre proliferation
- altered or increased sensory neuropeptide and inflammatory mediator expression
There is a ten-fold increase in bladder mast cell count in ulcer IC; however, in non-ulcer IC, the mast cell count is normal or only slightly increased. Other findings include:
- increased sympathetic innervation
- increased submucosal neuronal staining in IC
These findings lend support to a pathogenesis, which includes interaction between the inflammatory, neuronal and endocrine systems.
Urothelial dysfunction
Parsons and colleagues have proposed that a defective glycosaminoglycan (GAG) mucus layer in IC alters bladder permeability. Evidence supporting urothelial dysfunction includes widened tight junctions and increased permeability on scanning electron microscopy and the identification of APF by the urothelial cells in IC.
Autoimmune mechanisms
There is an association between IC and autoimmune diseases such as:
There are numerous reports on autoantibodies in some patients with IC.
Infection
No microorganism has been revealed as the cause of IC. There has been a large number of studies utilizing special techniques to detect microorganisms—including fastidious bacteria or viruses—which have been negative. There is no evidence of increased urinary IgA or IgG levels indicating recent or remote Gram-negative or Gram-positive infection in IC, and no evidence for helicobacter infection. It is speculated that a microbiological cause may be the initial trigger rather than being involved in the chronic process of IC.
Vascular changes
Vascular changes may play a role in the pathogenesis of IC. Decreased microvascular density in the suburothelium and bladder perfusion have been demonstrated to decrease with bladder filling in IC patients compared to the opposite in controls.
PATIENT RISK FACTORS
The only definitive risk factor for IC/PBS is female gender: the female-to-male ratio is generally estimated to be 9:1.2. However, in the managed care study just mentioned, the ratio was only 5:1.4. Other risk factors that have been proposed include heredity and previous urinary tract infection. Recent research suggests that heredity might play a role in the pathogenesis of IC/PBS.
Previous urinary tract infection has been proposed as a possible risk factor for IC/PBS. Anecdotal reports suggest that some patients experience the onset of IC/PBS symptoms after an episode of acute bacterial cystitis. However, current research does not support bacterial infection as a risk factor for the condition, although it remains possible that infection serves as a trigger in some patients.
- Race: Caucasians 90%
- Genetics: higher rate in first degree relatives
- Stress
- Associated psychological disorders: fibromyalgia or chronic fatigue syndrome
- History of childhood bladder problems
MANAGEMENT & THERAPY
General principles
Once a diagnosis of painful bladder disease has been made, patient education— including an overview of management options—is the next step.
Treatment is directed towards symptom reduction as there is no evidence for disease progression. A percentage of patients will spontaneously improve and ultimately resolve in weeks or months. Patients should be informed that IC/PBS is a non-progressive, non-malignant condition, and that there are many treatment options including expectant management.
Information regarding the large number of self-help strategies, natural therapies, and conservative and surgical options available means that patients feel they have greater control and choice with regard to their own treatment path. The Interstitial Cystitis Association and its affiliated international organizations are important resources for patients, researchers and clinicians.
Behavioural modification
A 1-day voiding chart gives both patient and clinician some objective measure of symptom severity and forms the basis of bladder re-education. Bladder training has been shown to increase voided volumes and improve frequency; however, the persistent sense of bladder fullness may still be a problem. Patients who experience pain more than frequency would be unlikely to tolerate bladder training as the sole intervention.
Physical therapy
Pelvic floor physical therapy is considered to have a place in the management of functional pelvic and perineal syndromes, but there are no published randomized, controlled trials to support this in IC. Biofeedback and soft tissue massage may aid in muscle relaxation of the pelvic floor. Some small uncontrolled series have reported success rates by varying techniques such as direct myofascial release, joint mobilization and home exercise program, transvaginal Theile massage and electromyographic biofeedback. One of the few randomized placebo-controlled studies performed
assessing this type of therapy in IC compared transdermal laser stimulation of the posterior tibial nerve to sham stimulation and found no difference between active and sham treatments.
Dietary manipulation
A significant proportion of interstitial cystitis patients have symptom exacerbation related to the intake of specific foods and beverages. Most often, these are ‘acidic’ beverages, coffee, spicy foods, and alcoholic beverages. However, the list of ‘foods to avoid’ has never been subjected to controlled clinical trials.
Oral therapy
The disease tends to fluctuate in severity, and up to 50% of patients experience temporary remissions unrelated to therapy for an average duration of 8 months.
A number of both oral treatments (such as L-arginine and Nalmefene) and intravesical therapies (such as hyaluronic acid and resiniferatoxin) have been shown not to be efficacious despite promising initial results.
Sodium pentosanpolysulfate/Elmiron: heparin analogue available in an oral formulation. The mechanism of action in IC is attributed to correction of a possible defect in the GAG layer, the ability to inhibit histamine release from mast cells, and a possible binding with inflammatory mediators in the urine. PPS has Food and Drug Administration approval for the treatment of the pain of interstitial cystitis.
Pain, urgency, and pressure showed significant improvement, but frequency, nocturia, and volume voided did not.
Elmiron is a well-tolerated medication and is efficacious in improving the pain of interstitial cystitis in up to one third of patients, often requiring a 3–6-month treatment duration to show an effect.
Amitriptyline: one of the most commonly used treatments for IC.
Amitriptyline has analgesic efficacy related to serotonin or noradrenaline reuptake inhibition and stabilizes mast cells. One quarter of patients in a trial setting were unable to tolerate the sedation accompanying 75 mg amitryptline daily. The sedation is helpful in the IC patient with bothersome night-time symptoms. A large multicenter trial to further investigate the efficacy and adverse effects of amitrypyline in IC is needed.
Hydroxyzine: a H1 receptor antagonist, inhibits bladder mast cell activation, has anticholinergic, angiolytic, and analgesic effects, and did not reach statistical significance.
Cimetidine: a H2 histamine receptor antagonist and has been studied mainly in openlabel series in the United Kingdom. A RCT of only 36 patients who received either oral cimetidine 400 mg twice daily or placebo showed an improvement in median
suprapubic pain and frequency scores.
IPD-1151T: Suplatast Tosilate (IPD-1151T) is an immunoregulator that suppresses IgE production and eosinophilia via suppression of helper T cells. It is used in Japan to treat allergic disorders such as asthma and atopic dermatitis. An initial small open-label study showed significantly increased bladder capacity and decreased urgency, frequency, and pain in 10 of 14 women.
Quercetin: a flavenoid with a potential anti-inflammatory effect. One open-label study in 22 patients for a duration of 1 month showed global improvement and amelioration of the O’Leary–Sant symptom and problem scores. Similar results were reported after 6-month open-label use of a combination of quercetin with chondroitin sulfate and sodium hyaluronate.
Antibiotics: significance was not reached in a RCT comparing a group of 25 patients receiving a total of 18-week sequence of rifampin, doxycycline, erythromycin, metronidazole, clindamycin, amoxicillin and ciprofloxacin versus 25 control patients. However, 80% of the antibiotic and 40% of the placebo group had adverse events—mainly nausea and/or vomiting and diarrhoea. It is common for many patients with IC to have been treated with empiric antibiotics, especially doxycycline. There is no evidence to support the use of antibiotics in the management of IC except in the presence of proven concurrent
infection.
Cyclosporine: eleven patients received cyclosporine for 3–6 months; frequency decreased, and mean and maximum voided volumes increased significantly. Bladder pain decreased or disappeared in 10 patients, but symptoms recurred after cessation of treatment in the majority. The short- and long-term adverse effects need to be carefully considered if cyclosporine therapy were used.
Analgesics: Medications commonly used for chronic neuropathic pain syndromes can be used for IC. These include antidepressants, anticonvulsants, non-steroidal anti-inflammatory drugs and antispasmodics. Opiates in this chronic pain state are most safely incorporated in the patient’s treatment in the setting of a multidisciplinary pain clinic, which allows for regular reassessment. Gabapentin has also been used in this setting, but no studies have been reported.
Small open-label series (10–25 patients) of drug therapy with methotrexate,
montelukast, nifedipine and misoprostol have been published showing some success, but no RCTs have been performed.
Intravesical therapy: grade B recommendation
Dimethyl sulfoxide: until 1996, the only drug approved by the FDA for use in IC. It is a chemical solvent with anti-inflammatory, analgesic, muscle relaxant, mast cell inhibition and collagen dissolution properties. DMSO can be administered as single therapy or in combination with heparin, steroids and a local anaesthetic as a cocktail. The treatment regimens vary to suit the convenience of patient and therapist, but once or twice weekly instillation for 8–12 treatments is commonly used. A response rate to DMSO of 50–90% has been reported, with a relapse rate of up to 40%; however, three quarters will respond to further DMSO. Adverse effects include a garlic-like breath odour and taste, and transient bladder irritability due to a chemical cystitis in 10%. DMSO is cheap, has relatively few side-effects, and self-instillation can be taught if required.
Intravesical therapy: grade C recommendation (beneficial, level 4 trials; majority opinion)
Heparin: two clinical uncontrolled studies of intravesical heparin 5000 U twice weekly have been reported that have shown improvement in bladder symptoms.
Lignocaine: several clinical studies of this drug in patients with interstitial cystitis have been reported. All involved electromotive drug administration (EMDA), utilizing an electric current to facilitate the active transport of the ionised drug. Short-term efficacy was demonstrated, but randomized controlled studies are needed. Other agents such
as oxychlorosene (chlorpactin) and silver nitrate have been used as instillations in the past but are rarely used now.
Intravesical therapy: grade A recommendation (not beneficial)
Bacillus Calmette–Guerin: two initial RCTs showed conflicting results.
Peters and colleagues demonstrated response to BCG in 60% of cases compared with 27% of controls; of the responders, eight of nine continued an excellent response at follow-upO2 years.
Peeker performed a cross-over RCT with BCG and DMSO and found that BCG was not efficacious.
Intravesical therapy: grade D recommendation (of no possible
recommendation)
Grade D is applicable to a number of intravesical therapies because the data are either
inadequate or conflicting. These drugs include capsaicin, oxybutynin, PPS and botulinum toxin type A.
Surgery for interstitial cystitis/painful bladder syndrome
Surgical options should be considered only when all conservative treatment has failed.
The consequences of surgical intervention—such as voiding dysfunction and the possibility of persistent pain and repeat surgery—should be discussed.
Sacral neuromodulation consists of a two-stage procedure involving temporary or percutaneous sacral S3 or S4 root stimulation followed by permanent implantation in responders. Sacral nerve modulation is a promising surgical treatment for IC/PBS; however, more information is needed on long-term follow-up, adverse events and reoperation rates.

Both cystolysis or peripheral denervation surgery and sympathetic/parasympathetic denervation have been associated with long-term symptom recurrence in addition to voiding dysfunction. These procedures are not indicated for IC/PBS.
Bladder augmentation or cystoplasty has been used for refractory IC/PBS for many years. First reports of ileocystoplasty were very good, but later publications were variable with good results varying from 25 up to 100%. Cystoplasty with subtrigonal cystectomy (trigone removal but preservation of the bladder neck) has no advantage over supratrigonal cystectomy and is associated with complications related to the need for ureteric reimplantation such as anastomotic leak, stricture or reflux as well as voiding dysfunction requiring self-catheterization.
 p=.
p=. 
Total cystectomy and urethrectomy is the ultimate option with simple or continent urinary diversion. Lotenfoe described best results (88%) in older patients with reduced capacity <400 mL compared with 20% in patients with capacity >400 mL.
Unfortunately, pelvic pain can persist even after such major surgery.

COMPLICATIONS
- Women with IC have significantly more FSD (female sexual dysfunction) and sexual distress than women without IC review
- Sexual dysfunction in patients with bladder syndrome is age related and progressive review
- Patients with IC were 100 times more likely to have inflammatory bowel disease and 30 times more likely to have systemic lupus erythematosus
- No evidence exists that IC/PBS increases the risk of bladder cancer