Hot flashes (also known as hot flushes, or night sweats if they happen at night) are a symptom of the changing hormone levels that are considered to be characteristic of menopause
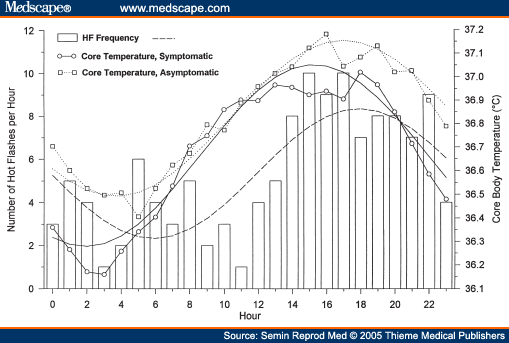
Pathophysiology and Treatment of Menopausal Hot Flashes: Circadian Rhythm of Hot Flashes
Hot flashes and blood pressure in midlife women. 2010
Maturitas. 2010 Jan;65(1):69-74. Epub 2009 Nov 28.
Gallicchio L, Miller SR, Zacur H, Flaws JA.
OBJECTIVES: Recent epidemiological studies suggest that hot flashes may have a detrimental impact on the cardiovascular system. The purpose of this study was to examine the associations between hot flashes and blood pressure among women aged 45-54 years who had never used hormone therapy. STUDY DESIGN: Data were analyzed from 603 women who participated in the Midlife Health Study, a cross-sectional study conducted in the Baltimore Metropolitan region. MAIN OUTCOME MEASURES: All participants came to the clinic where systolic and diastolic blood pressures were measured, height and weight were assessed, and a questionnaire was administered that ascertained detailed data on history of hot flashes and participant demographics and health habits. RESULTS: The data showed that 56.9% of the participants reported ever experiencing hot flashes. In the age-adjusted analyses, both systolic and diastolic blood pressures were significantly and positively associated with hot flashes. However, the estimates were markedly attenuated and not statistically significant after adjustment for age, race, smoking status, current alcohol use, body mass index, and use of an anti-hypertensive agent or a cholesterol-lowering medication. Similar results were observed for moderate or severe hot flashes, hot flashes experienced for one or more years, and hot flashes experienced within the previous 30 days. CONCLUSIONS: These findings indicate that hot flashes are not significantly associated with blood pressure during midlife.
Hot flashes are associated with increased ambulatory systolic blood pressure., 2007
Menopause. 2007 Mar-Apr;14(2):308-15.
Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE.
Department of Public Health, Hypertension Center, Weill Medical College of Cornell University, New York, NY 10021, USA. LIG2002@med.cornell.edu
OBJECTIVE: To determine the association between ambulatory blood pressure (BP) and hot flash experience. DESIGN: The participants in the study were 154 women (mean age=46 years, range=18-65 years), who were evaluated as part of a cross-sectional study on ethnicity, socioeconomic status, and diurnal BP patterns. Participants could be either normotensive or mildly hypertensive. Participants wore an ambulatory BP monitor for 24 hours and recorded their awake and sleep times. Hot flashes were assessed using an everyday complaint questionnaire that embeds symptoms associated with menopause into a list of everyday complaints. RESULTS: Thirty-three percent of participants reported having had hot flashes during the 2 weeks before they completed the questionnaire. Compared with women who did not report hot flashes, mean awake and sleep systolic BP values were significantly higher (P<0.004 and P=0.007, respectively) in women who reported having had hot flashes. Hot flashes continued to independently predict average awake and sleep systolic BP (both P=0.03) after controlling for age, race/ethnicity, body mass index, and menopausal status. Hot flashes were not associated with diastolic BP or nocturnal dipping of BP. CONCLUSIONS: Hot flashes are associated with increased awake and sleep systolic BP independent of menopausal status. Further investigation is warranted to elucidate the mechanisms by which hot flashes are associated with BP.
Cortisol levels during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women's Health Study. 2009
Menopause. 2009 Jul-Aug;16(4):708-18.
Woods NF, Mitchell ES, Smith-Dijulio K.
OBJECTIVE: Cortisol levels rise among some women during the late stage of the menopausal transition (MT), but we know little about changes in cortisol levels in relation to menopause-related factors (MT stage, urinary estrone glucuronide [E1G], testosterone, follicle-stimulating hormone [FSH]), stress-related factors (epinephrine, norepinephrine, and perceived stress), symptoms (hot flashes, mood, memory, and sleep), social factors (income adequacy, role burden, social support, employment, parenting, and history of sexual abuse), and health-related factors (depressed mood, perceived health, physical appraisal, body mass index, and smoking). The aim of the study was to examine the influence of menopause-related factors, stress-related factors, symptoms, social factors, and health-related factors on cortisol levels during the MT. METHODS: Participants were a subset of Seattle Midlife Women's Health Study who provided data during the late reproductive, early and late MT stages, or early postmenopause and who were not using hormone therapy or corticosteroids (N = 132 women, up to 5,218 observations). Data provided included menstrual calendars for staging the MT, annual health reports, health diaries, and overnight urine specimens (assayed for cortisol, catecholamines, E1G, and FSH) between 1990 and 2005 were included. Perceived stress, symptoms, and health behaviors were assessed in a health diary. Health-related and social factors were assessed in an annual health update. Multilevel modeling was used to test the effects of menopause-related and other factors on overnight cortisol levels. RESULTS: When tested with age as a measure of time, menopause-related covariates, including E1G, FSH, and testosterone, were associated with significant increases in overnight cortisol levels (P < 0.0001). Likewise, epinephrine and norepinephrine were each associated significantly with overnight cortisol levels (P < 0.0001). In multivariate analyses, E1G, FSH, and testosterone constituted the best set of predictors. CONCLUSIONS: Overnight cortisol levels during the MT were associated with E1G, testosterone, and FSH levels. In addition, they were significantly and positively associated with epinephrine and norepinephrine. MT stage, symptoms, and social, stress-related, and health-related factors had little relationship to overnight cortisol levels when other biological indicators were considered.
Biophysical and endocrine-metabolic changes during menopausal hot flashes: increase in plasma free fatty acid and norepinephrine levels., 1989
Gynecol Obstet Invest. 1989;27(1):34-7.
Cignarelli M, Cicinelli E, Corso M, Cospite MR, Garruti G, Tafaro E, Giorgino R, Schonauer S.
Thermocutaneous, vascular, metabolic and hormonal changes were investigated during 11 hot flashes from 6 postmenopausal women. The first detectable change was an increase in finger blood flow with a concomitant enhancement of skin conductance. The increase in skin conductance was followed rapidly by a sharp rise in finger temperature. The main endocrine-metabolic changes associated with the above phenomena were a sharp increase in plasma free fatty acids (approximately 65%), norepinephrine (approximately 100%) and LH (approximately 20%) levels. Plasma glucose and cortisol tended to be increased but did not reach statistical significance; on the other hand, plasma insulin, glucagon, growth hormone, epinephrine and dopamine remained unchanged.
Pituitary hormones during the menopausal hot flash. 1984
Obstet Gynecol. 1984 Dec;64(6):752-6.
Meldrum DR, Defazio JD, Erlik Y, Lu JK, Wolfsen AF, Carlson HE, Hershman JM, Judd HL.
Eighteen postmenopausal women with severe hot flashes had continuous recordings of finger temperature and skin resistance as objective indexes of flushing episodes, and serial measurements of anterior pituitary hormones as indirect indexes of hypothalamic neurotransmitter activity. Significant increases of growth hormone, adrenocorticotropic hormone (ACTH), and luteinizing hormone (LH) occurred with maximal concentrations at 30, five, and 15 minutes, respectively, after the onset of the skin temperature rises. No significant fluctuations of prolactin (PRL), thyroid-stimulating hormone (TSH), or follicle-stimulating hormone (FSH) were observed. The mean serum cortisol concentration increased 15 minutes after the hot flash, presumably consequent to the preceding elevation of ACTH. Pituitary ACTH release may be secondary to hypothalamic cooling, whereas increased growth hormone and LH output and the thermoregulatory adjustments comprising the flushing episodes are all consistent with cyclic episodes of increased hypothalamic norepinephrine activity.
The effect of clonidine on pituitary hormone secretion in physiological and pathological states. 1987
J Cardiovasc Pharmacol. 1987;10 Suppl 12:S235-9.
Baranowska B.
The purpose of this study was to evaluate the effect of clonidine--an alpha 2-adrenergic agonist--and naloxone--an opiate antagonist--on pituitary hormone release. The study involved 43 women: 20 menopausal women, 9 untreated women with ACTH-dependent Cushing's disease, and 14 healthy women. Serum GH, ACTH, LH, FSH, TSH, cortisol, and plasma beta-endorphin concentrations were measured with RIA methods. A significant increase in GH and a significant decrease in ACTH and in cortisol was observed after clonidine injection in healthy women. Clonidine caused a significant decrease in LH concentration in the luteal phase of the menstrual cycle. However, naloxone induced the opposite effect on pituitary hormone release. In Cushing's disease, ACTH significantly decreased in response to clonidine. In postmenopausal women with hypertension a decrease in blood pressure, a marked decrease in the number of hot flashes, as well as a diminution in amplitude and frequency of LH pulsatility was found. Conclusions are as follows: (1) Clonidine may be useful in the treatment of hypertensive menopausal women; and (2) a diminution in ACTH, beta-endorphin, and cortisol release in response to clonidine was observed in Cushing's disease.
The on-off switches of the mitochondrial uncoupling proteins. 2009
Trends Biochem Sci. 2009 Dec 16. [Epub ahead of print]
Azzu V, Brand MD.
MRC Mitochondrial Biology Unit, Hills Road, Cambridge CB2 0XY, UK.
Mitochondrial uncoupling proteins disengage substrate oxidation from ADP phosphorylation by dissipating the proton electrochemical gradient that is required for ATP synthesis. In doing this, the archetypal uncoupling protein, UCP1, mediates adaptive thermogenesis. By contrast, its paralogues UCP2 and UCP3 are not thought to mediate whole body thermogenesis in mammals. Instead, they have been implicated in a variety of physiological and pathological processes, including protection from oxidative stress, negative regulation of glucose sensing systems and the adaptation of fatty acid oxidation capacity to starving. Although much work has been devoted to how these proteins are activated, little is known of the mechanisms that reverse this activation. Copyright © 2009 Elsevier Ltd. All rights reserved.
Uncoupling protein-3 as a molecular determinant of the action of 3,5,3'-triiodothyronine on energy metabolism. 2009
Endocrine. 2009 Oct;36(2):246-54. Epub 2009 Jul 14.
Flandin P, Lehr L, Asensio C, Giacobino JP, Rohner-Jeanrenaud F, Muzzin P, Jimenez M.
Thyroid hormones are known to stimulate thermogenesis in rodents by exerting a permissive effect on norepinephrine that affects uncoupling protein-1 (UCP1) expression in brown adipose tissue (BAT). The aim of this study was to identify new targets of the thermogenic effects of T3 in tissues other than the BAT, such as skeletal muscle. In beta(1)/beta(2)/beta(3)-adrenoceptor knockout (beta-less) mice, that are dramatically cold intolerant, a normal body temperature was maintained throughout 48 h of cold exposure by T3 administration. In these mice, BAT UCP1 protein expression was not modified either by cold exposure or by T3 administration. To test the possibility that T3 might act via muscle uncoupling protein-3 (UCP3), an UCP3 knockout (KO) model was used. This model exhibited a normal phenotype except that, upon T3 administration, stimulated oxygen consumption of the UCP3KO mice was significantly lower by 6% than that of the wild-type (WT) mice. This difference was observed only during the dark period (between 7.00 p.m. and 7.00 a.m.), i.e. when the mice are the most active at consuming food. Therefore, UCP3 might participate in the correction by T3 of the dramatic cold intolerance of the beta-less mice. These results reactivate the idea that UCP3 might play a role in the control of energy balance.
Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. 2010
Diabetes. 2010 Jan;59(1):17-25. Epub 2009 Oct 15.
Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, Xiao F, Guo F.
OBJECTIVE: White adipose tissue (WAT) and brown adipose tissue (BAT) play distinct roles in adaptation to changes in nutrient availability, with WAT serving as an energy store and BAT regulating thermogenesis. We previously showed that mice maintained on a leucine-deficient diet unexpectedly experienced a dramatic reduction in abdominal fat mass. The cellular mechanisms responsible for this loss, however, are unclear. The goal of current study is to investigate possible mechanisms. RESEARCH DESIGN AND METHODS: Male C57BL/6J mice were fed either control, leucine-deficient, or pair-fed diets for 7 days. Changes in metabolic parameters and expression of genes and proteins related to lipid metabolism were analyzed in WAT and BAT. RESULTS: We found that leucine deprivation for 7 days increases oxygen consumption, suggesting increased energy expenditure. We also observed increases in lipolysis and expression of beta-oxidation genes and decreases in expression of lipogenic genes and activity of fatty acid synthase in WAT, consistent with increased use and decreased synthesis of fatty acids, respectively. Furthermore, we observed that leucine deprivation increases expression of uncoupling protein (UCP)-1 in BAT, suggesting increased thermogenesis. CONCLUSIONS: We show for the first time that elimination of dietary leucine produces significant metabolic changes in WAT and BAT. The effect of leucine deprivation on UCP1 expression is a novel and unexpected observation and suggests that the observed increase in energy expenditure may reflect an increase in thermogenesis in BAT. Further investigation will be required to determine the relative contribution of UCP1 upregulation and thermogenesis in BAT to leucine deprivation-stimulated fat loss.
Hot flashes and cardiac vagal control: a link to cardiovascular risk? 2009
Menopause () (2009)
profile Rebecca C Thurston, profile Israel C Christie and profile Karen A Matthews
From the 1Department of Psychiatry, School of Medicine, and 2Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA.
OBJECTIVE:: The understanding of the physiology of hot flashes is incomplete. The autonomic nervous system has been hypothesized to play a role in hot flashes but has received limited empirical attention. Furthermore, emerging research has linked hot flashes to cardiovascular risk. Reduced high-frequency heart rate variability (HF-HRV), an index of vagal control of heart rate, has been associated with cardiovascular events. We hypothesized that decreases in HF-HRV would occur during hot flashes relative to periods before and after hot flashes. METHODS:: Thirty perimenopausal and postmenopausal women aged 40 to 60 years reporting four or more hot flashes per day underwent laboratory hot flash provocation testing, with electrocardiogram and measurement of sternal skin conductance. Hot flashes were reported and identified from sternal skin conductance. HF-HRV was estimated using spectral analysis of the heart rate time series. The 5-minute interval during the hot flash period was compared with that during two nonflash periods before and after the hot flash via mixed-effects models. RESULTS:: HRV was significantly decreased during hot flashes relative to periods before (b = 0.18, SE = 0.05; P = 0.0001) and after (b = 0.16, SE = 0.05; P = 0.002) physiologically measured hot flashes, controlling for age, race, education, task condition, menopause status, task, hypertension status, diabetes status, physical activity, body mass index, smoking, and anxiety. Findings were unchanged when considering self-reported hot flashes. CONCLUSIONS:: Significant decreases in cardiac vagal control occurred during hot flashes, which may help shed light on the physiology of hot flashes. The autonomic nervous system may deserve greater attention in understanding the mechanisms linking hot flashes to cardiovascular risk. | PMID: 20042892 | DOI: 10.1097/gme.0b013e3181c7dea7