Written by: Mattia Brunasso, Luca Debernardi, Filippo Crivelli
DEFINITION
Tetanus is a deadly disease of warm blooded animals and humans characterized by a prolonged contraction of skeletal muscle fibers.
Tetanus is classified into four type:
- Generalised tetanus is the most common presentation in which muscles throughout the body are affected, with the head and neck being usually affected first followed by a caudal spread of spasms.
- Localised tetanus occurs if the rigidity and pain remain localized to the site of injury, and is usually associated with a better prognosis.
- Cephalic tetanus following a head or neck injury, and which is associated with high mortality. The seventh cranial nerve is the most involved, but also the oculomotor nerve may be affected.
- Neonatal tetanus is rare in the developed world. It involved babies born to a mother who did not possess enough circulatory antitoxin to protect the fetus passively by transplacental transfer. The condition arises also from poor umbilical hygiene, and is prevalent in communities that employ traditional midwifery practices such as cutting the cord with grass or dirty scissors, or rubbing manure on the umbilical stump.
EPIDEMIOLOGY
Tetanus is caused by an exotoxin called tetanospasmin or tetanus neurotoxin (TeNT) produced by bacterium named Clostridium tetani. C.tetani is a gram positive, obligate anaerobic spore forming bacillus. It’s found as spores in soil or in the gastrointestinal tract of animals and can also be found on human skin. During vegetative growth, the organism cannot survive in the presence of oxygen, is heat-sensitive and exhibits flagellar motility. The spores are extremely stable, and although boiling for 15 minutes kills most, some will survive unless autoclaves at 120° C, 1.5 bar, for 15 minutes, which ensure sterility.

The infection occurs through the contamination of lacerated-contused wounds, where in conditions of low oxygen tension the spores germinate and the resultant bacteria multiply and produce toxin responsible for the clinical features of tetanus.
SYMPTOMS
Tetanus typically follows deep penetrating wounds where anaerobic bacterial growth is facilitated. The most common portals of infection are wounds on the lower limbs, post-partum or post-abortion infections of the uterus, non-sterile intramuscular injections, and compound fractures. However, even minor trauma can lead to disease and in up to 30% of patients no portal of entry is apparent. Tetanus has been reported after a myriad of injuries, including intravenous and intramuscular injections, acupuncture, ear piercing, and even from toothpicks. The incubation period (the time from inoculation to the first symptom) can be as short as 24 hours or as long as many months after inoculation with C. tetani. This interval is a reflection of the distance the toxin must travel within the nervous system, and may be related to the quantity of toxin released. The period of onset is the time between the first symptom and the start of spasms. These periods are important prognostically, the shorter the incubation period or period of onset the more severe the disease.
- Trismus (lockjaw), the inability to open the mouth fully owing to rigidity of the masseters, is often the first symptom.
Other obvious symptoms may be:
- risus sardonicus (typical expression due to the spastic paralysis of facial muscles );
- stiffness, tightness or spasm about the mouth or jaws which made it difficult to eat, swallow or to talk;
- fever and irregular heartbeat;
- severe abdominal pain caused by violent spasms of the musculature of the anterior abdominal wall;
- chest retrosternal pain;
- right inferior oblique muscle weakness (cranial nerve paralysis);
- reduced right corneal reflex (cranial nerve paralysis );
- right facial paralysis;
- dysphagia;
- abnormal tongue movements;
- limbs paralysis.

When local tetanus occurs from head and facial injuries cephalic tetanus can develop, which is a local variant but has a higher mortality. Generalised tetanus is the most common form of the disease, and presents with:
- pain;
- headache;
- stiffness;
- rigidity;
- opisthotonus;
- spasms, which can lead to laryngeal obstruction.
The spasms are extremely painful and may be
leading to respiratory arrest and death. Spasms are most prominent in the first 2 weeks; autonomic disturbance usually starts some days after spasms and reaches a peak during the second week of the disease. Rigidity may last beyond the duration of both spasms and autonomic disturbance. Severe rigidity and muscle spasm necessitates
paralysis for prolonged periods in severe tetanus.

DIAGNOSIS
The diagnosis is a clinical one, relatively easy to make in areas where tetanus is seen often, but often delayed in the developed world where cases are seen infrequently. The differential includes tetany, strychnine poisoning, drug induced dystonic reactions, rabies, and orofacial infection. In neonates the differential diagnosis would also include hypocalcaemia, hypoglycemia, meningitis, meningoencephalitis, and seizures.
TETANUS TOXIN AND PATHOGENESIS

Tetanospasmin is an A-B toxin. The toxin gene is encoded on a 75 kb plasmid and synthesized as a single polypeptide with a molecular weight of 150 000. The polypeptide undergoes post-translational cleavage into two disulfide linked fragments, the light (LC or A) and heavy (HC or B) chains, respectively of 50 kDa and 100 kDa. The carboxyl terminal portion of the H chain, termed Hc, which is composed of two ~ 25 kDa domains, a lectin like jelly role domain (Hcn) and a β-trefoil domain (Hcc), mediates attachment to gangliosides (GD1b and GT1b) on the surface of nonmyelinated nerve terminals, and subsequently the toxin is endocytosed.
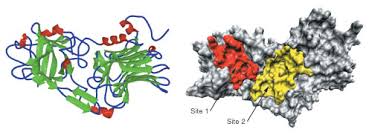
xRay crystallographic studies have shown the structural homology of these two domains with proteins such as interleukin (IL-1α) and may reflect the ability of the Hc fragment to bind to receptors. Ligand–receptor complexes are internalized by a variety of endocytosis mechanisms. Some are initiated within clathrin-coated membranes, whereas others involve lipid microdomains of the plasma membrane. In neurons, where alternative targeting to short- or long-range trafficking routes underpins the differential processing of synaptic vesicle components and neurotrophin receptors; the mechanism giving access to the axonal retrograde pathway remains unknown. It is demonstrated that TeNT HC internalization also relies on a specialized clathrin-mediated pathway, which is independent of synaptic vesicle recycling. Moreover, unlike transferrin uptake, the process of internalization of TeTN is independent of epsin1.

These findings identify a pathway for TeNT, beginning with the binding to a lipid raft component (GD1b) and followed by dissociation from GD1b as the toxin internalizes via a clathrin-mediated mechanism using a specific subset of adaptor protein. It is then moved from the peripheral nerves to the central nervous system by retrograde axonal transport (mediated by ATPase dynein-dependent) and trans-synaptic spread. The entire toxin molecule is internalized into presynaptic cells (for example cell of Renshaw) and the toxin becomes exposed to an acidic environment. Acidification triggers a structural rearrangement in Hn (the N-terminus of HC, composed of a largely α-helical domain of ~ 50 kDa) and the formation of a cation selective channel. This is probably coupled to partial unfolding of the LC, its entry into and transit through the Hn channel followed by its refolding in the cytosol. Subsequent to reduction of the disulfide bridge the LC is released from the HC. Once in the cytosol the LC exerts its catalytic activity. The L chain is a zinc metalloprotease, which cleaves synaptobrevin, an integral membrane component, essential for the fusion of synaptic vesicles with the presynaptic membrane. Cleavage by toxin tetanus L chain prevents release of their contents, GABA and particularly glycine, the most important spinal inhibitory neurotrasmitter, into the synaptic cleft from inhibitory interneuron. The α- motoneurons are therefore under no inhibitory control and undergo sustained excitatory discharge causing the characteristic motor spasms of tetanus.


The toxin exerts its effect on the spinal cord, the brain stem, peripheral nerves, at neuromuscular junctions, and directly on muscles. To what extent cortical and subcortical structures are involved remains unknown.
CLEAVAGE OF SYNAPTOBREVIN
Synaptobrevin, also termed VAMP (vesicle associated membrane protein) is an integral membrane protein which belongs to the family of SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) proteins. A common feature of all SNAREs is the presence of one α-helical domain of ~70 amino acids, the SNARE motif, endowed with the capacity to form coiled-coils. The function of these proteins is to mediate the docking of vesicles with the membrane. The best know SNARE proteins are those involved in neurotrasmitters release. The SNARE proteins relevant to the regulated exocytosis are: synaptobrevin/VAMP (localized on the vesicular membrane), syntaxin-1 and the SNAP-25 (both localized on the presynaptic plasma membrane) which together form a protein complex called SNARE complex. In the presynaptic active zone, the increase in calcium due to the influence of calcium through VDCC (voltage-dependent calcium channels), recognized by synaptotagmin which is a calcium sensor, is the signal that triggers the fusion of synaptic vesicles. The cast is led by the progressive anchoring of these three proteins to form bundles. Each beam is formed by two α-helices of SNAP-25, one of syntaxin-1 and one of synaptobrevin. The bundles mediate the fusion between the two membrane and consequently the release of neurotrasmitter. The intracellular mode of action of the neurotoxin was discovered in the early 1990s, initiated by the identification on Zn2+ binding motif, His-Glu-X-X-His, in the primary sequence of TeNT. Shortly after, selective searches for substrates among synaptic vesicles proteins led to the identification of the integral synaptic vesicle membrane protein VAMP as a target for TeNT. The cleaving of synaptobrevin from vesicle inhibits the process of membranes fusion and of course the release of glycine. This results in the blockade of neurotransmission.

1."Mechanism of action of tetanus and botulinum neurotoxins." 1994: http://www.ncbi.nlm.nih.gov/pubmed/7527117
2."Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses." 1999: http://www.ncbi.nlm.nih.gov/pubmed/10212474
3."How botulinum and tetanus neurotoxins block neurotransmitter release." 2000: http://www.ncbi.nlm.nih.gov/pubmed/10865130
4."Gangliosides as high affinity receptors for tetanus neurotoxin." 2009: http://www.ncbi.nlm.nih.gov/pubmed/19602728
5."Human Monoclonal ScFv That Inhibits Cellular Entry and Metalloprotease Activity of Tetanus Neurotoxin" 2010: http://www.ncbi.nlm.nih.gov/pubmed/20527521
RISK FACTORS
Tetanus was first described in Egypt over 3000 years ago and was prevalent throughout the ancient world. Despite the availability of passive immunization since 1893 and an effective active vaccination since 1923, tetanus remains a major health problem in the developing world and is still encountered in the developed world. There are between 800 000 and 1 million deaths due to tetanus each year. Eighty per cent of these deaths occur in Africa and south east Asia and it remains endemic in 90 countries world wide. Incomplete vaccine deployment among the population at risk is the major factor.

COMPLICATIONS
Respiratory failure is the commonest direct cause of death from tetanus in the developing world, particularly when artificial ventilation may not be available for every case. Where it is available attempts should be made to anticipate and detect patients at risk from hypoxia and airways obstruction, aspiration hypoventilation, pneumonia, and respiratory arrest. Early airways protection and ventilatory support is often needed.
- Autonomic nervous system disturbance with sustained labile hypertension, tachycardia, vasoconstriction, and sweating is common in severe cases. Profound bradycardia and hypotension may occur and may be recurrent or preterminal events. Involvement of the sympathetic nervous system was recognised in 1968. Sedation, which is useful for controlling spasms and rigidity, is also the first step in reducing autonomic instability: benzodiazepines are the most commonly used sedative agents (they augment GABA agonism by inhibiting an endogenous inhibitor at the GABA-receptor). Adequate sedation is essential in tetanus.
- Sometimes the patient may present fractures or tendon injuries due to abnormal contractile spasms.
THERAPY
Penicillin remains the standard therapy for tetanus in most parts of the world, although antibiotics for Clostridium tetani probably play a relatively minor part in the specific treatment of the disease. The dose is 100 000–200 000 IU/kg/day intramuscularly or intravenously for 7 to 10 days. The structure of penicillin, distant to the β-lactam ring, is similar to aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS. Penicillin therefore acts as a competitive antagonist to GABA. Penicillin does not readily cross the blood-brain barrier, but in high cumulative doses it can cause CNS hyperexcitability. In tetanus this side effect of penicillin could synergize with the action of the toxin in blocking transmitter release at GABA neurons. Therefore penicillin is dangerous for causing convulsions (it is used for induction of local experimental epilepsy). Metronidazole is a safe alternative, and may now be considered as the first line therapy. After rectal administration metronidazole is rapidly bioavailable and causes fewer spasms than repeated intravenous or intramuscular injections. If metronidazole is available and applicable this should be considered as the drug of choice in the treatment of tetanus. The dose is 400 mg rectally every 6 hours, or 500 mg every 6 hours intravenously for 7–10 days. Pyridoxine (vitamin B6) is a coenzyme with glutamate decarboxylase in the production of GABA from glutamic acid, and increases GABA concentrations in animal models. However, its role in the management of tetanus has not been confirmed yet.
VACCINATION
Passive immunisation with human or equine tetanus immunoglobulin shortens the course and may reduce the severity of tetanus. The human antiserum is isolated from a pool of plasma derived from healthy human tetanus immune donors, and has a half-life of 24.5–31.5 days. The equine (or bovine) form, widely available throughout the developing world, has a higher incidence of anaphylactic reactions and a half life of only 2 days, but is much cheaper to produce. If available, human antiserum should be administered but in most parts of the world equine antitoxin is the standard.
In established cases patients should receive 500–1000 IU/kg equine antitoxin intravenously or intramuscularly. Anaphylactic reactions occur in 20% of cases; in 1% they are severe enough to warrant adrenaline, antihistamines, steroids, and intravenous fluids. If available 5000–8000 IU human antitetanus immunoglobulin should be given intramuscularly: this has a lower incidence of side effects. Active vaccination leads to long-term humoral and cellular immunity. Tetanus toxoid for vaccination is produced by formaldehyde treatment of the toxin and its immunogenicity is improved by absorption with aluminium hydroxide. Aluminium hydroxide absorbed tetanus toxoid is very effective at preventing tetanus with a failure rate of 4/100 million immunocompetent people. Serum antitoxin concentrations above 0.01U/ml are considered protective, although there have been patients reported with protective serum antibody concentrations. A protective antibody concentration is attained after the second dose, but a third dose ensures longer lasting immunity. To maintain adequate concentrations of protection additional booster doses should be administered every 10 years. Reactions to the tetanus toxoid are estimated to be 1 in 50 000 injections, although most are not severe; local tenderness, oedema, flu-like illness, and low-grade fever are the most often encountered. Neonatal tetanus can be prevented by immunisation of women during pregnancy.
BIBLIOGRAPHY
1. Tetanus
2. "Toxigenic Clostridia" 1990: http://www.ncbi.nlm.nih.gov/pubmed/2404569
3."Patogenic Clostridia, Including Botulism and Tetanus": http://textbookofbacteriology.net/clostridia_4.html