INTRODUCTION
Human papillomavirus (HPV) is one of the most common causes of sexually transmitted disease in both men and women worldwide and is thought to be the most common sexually transmitted viral disease in the United States.
Genital HPV infection is not a reportable disease, so actual incidence and prevalence figures are not known; however, it is estimated that the incidence of new infections in the United States ranges from 1 million to 5.5 million per year, and the prevalence is estimated to be as high as 20 million (1 Cates, W., and the American Social Health Association Panel. 1999. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex. Transm. Dis. 26:S2-S7. [PubMed]). HPV continues to be an important topic, as rates of infection appear to continue to be rapidly increasing.
Papillomaviruses are ubiquitous and have been detected in a wide variety of animals as well as in humans and are specific for their respective hosts.
More than 200 types of HPV have been recognized on the basis of DNA sequence data showing genomic differences. Eighty-five HPV genotypes are well characterized. An additional 120 isolates are partially characterized potential new genotypes (2 zur Hausen, H. 1999. Papillomaviruses in human cancers. Proc. Assoc. Am. Physicians 111:581-587. [PubMed]).
HPVs can infect basal epithelial cells of the skin or inner lining of tissues and are categorized as cutaneous types or mucosal types:
-Cutaneous types of HPV are epidermitrophic and target the skin of the hands and feet.
-Mucosal types infect the lining of the mouth, throat, respiratory tract, or anogenital epithelium.
Based on their association with cervical cancer and precursor lesions, HPVs can also be grouped to high-risk and low-risk HPV types:
low risk hpv types include types 6, 11, 42, 43, and 44.
high risk hpv types include types 16, 18, 31, 33, 34, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 70. Included in the high-risk group are some HPV types that are less frequently found in cancers but are often found in squamous intraepithelial lesions (SILs)
| disease | HPV type |
|---|
| plantar warts | 1,2,4,63 |
| common warts | 2,1,7,4,26,27,29,41,57,65 |
| flat warts | 3,10,26,27,28 |
| other cutaneous lesions | 6,11,16,30,33 |
| epidermodysplasia verruciformis | 2,3,10,5,8,9,12,14,15,17 |
| recurrent respiratory papillomatosis | 6,11 |
| condyloma acuminata (genital warts) | 6,11,30,42,43,45,51,54,55,70 |
| cervical intraepithelial neoplasia |
| unspecified | 30,34,39,40,53,57,59,61,62,64,66,67,68,69 |
| low risk | 6,11,16,18,31,33,35,42,43,44,45,51,52,74 |
| high risk | 16,18,6,11,31,34,33,35,39,42,44,45,51,52,56,58,66 |
| cervical carcinoma | 16,18,31,45,33,35,39,51,52,56,58,66,68,70 |

TREATMENT
Most HPV-induced cervical cell changes are transient, and 90% regress spontaneously within 12 to 36 months as the immune system eliminates the virus (3 Chua, K. L., and A. Hjerpe. 1996. Persistence of human papillomavirus (HPV) infections preceding cervical carcinoma. Cancer 77:121-127. [PubMed]
4 Ostor, A. G. 1993. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol. 12:186-192. [PubMed]).
The primary immune response to HPV infection is a cell-mediated response induced at local lymph nodes.
A humoral immune response also develops, but local levels of HPV-specific immunoglobulin G (IgG) and IgA in tissue do not correlate with clearance of virus (5 Bontkes, H. J., T. D. deGruijl, J. M. Walboomers, J. T. Schiller, J. Dillner, T. J. Helmerhorst, R. H. Vereheijen, R. J. Scheper, and C. J. Meijer. 1999. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J. Gen. Virol. 80:409-417. [PubMed]).
Systemic levels of HPV-specific IgA were correlated with virus clearance.
Systemic levels of HPV-specific IgG were detected more frequently in patients with persistent HPV infection.
The tendency toward regression of HPV infection correlates inversely with the severity of cervical disease.
Only a small proportion of mild and moderate cervical diseases develop into invasive cancer, but the risk of progression from severe cervical cellular abnormality to invasive carcinoma is at least 12% (4.).
Factors such as genetic predisposition, frequency of reinfection, intratypic genetic variation within HPV type, coinfection with more than one HPV type, hormone levels, and immune response may influence the ability to clear an HPV infection.
A number of factors such as size, stage, and histologic features of the tumor, lymph node involvement, risk factors for complications from surgery or radiation, and patient preference determine the course of treatment.
In general, noninvasive intraepithelial lesions identified only microscopically are treated with superficial ablative procedures such as cryotherapy or laser therapy.
These are outpatient office procedures, and fertility is maintained.
With cryotherapy, abnormal tissue and the surrounding 5 mm is frozen with a supercooled probe. A single freeze is usually not adequate to induce necrosis, so the area is allowed to thaw and is frozen again.
Ablation of tissue with a carbon dioxide laser beam is as effective as cryotherapy, and the tissue heals faster with less distortion, but the procedure is more expensive.
Loop electrosurgical excision procedures are now considered to be the preferred treatment for noninvasive squamous lesions. In these procedures, an electrically charged wire is used to excise the transformation zone and distal endocervical canal. It is less expensive than laser therapy and preserves the excised tissue for histologic examination of margin status.
Following treatment of noninvasive intraepithelial neoplasia lesions by any technique, there is always a potential risk of leaving dysplastic cells behind. Recurrence rates as high as 31% with a mean time to recurrence of 11.9 months have been reported following loop diathermy procedures in immunologically normal patients (6 Gonzalez, D. I., C. M. Zahn, M. G. Retzloff, W. F. Moore, E. R. Kost, and R. R. Snyder. 2001. Recurrence of dysplasia after loop electrosurgical excision procedures with long-term follow-up. Am. J. Obstet. Gynecol. 184:315-321. [PubMed]).
Patients with positive margins had a higher recurrence rate (47%) than did those with clear margins (26%). Human immunodeficiency virus-infected women have a significantly higher recurrence rate (87%) than do uninfected women (18%), indicating the importance of an effective immune system in resolution of HPV-associated disease (7 Calore, E. E., S. M. M. Pereira, and M. J. Cavaliere. 2001. Progression of cervical lesions in HIV-seropositive women: a cytological study. Diagn. Cytopathol. 24:117-119. [PubMed]).
Progression to invasive disease is rare (< 2% in most series).
However, these data emphasize the importance of follow-up surveillance in treated patients. Preliminary evidence suggests that detection of HPV DNA using molecular techniques may be able to help detect residual lesions following treatment (8 Nobbenhuis, M. A., C. J. Meijer, A. J. van den Brule, L. Rozendaal, F. J. Voorhoost, E. K. Risse, R. H. Verheijen, and T. J. Helmerhorst. 2001. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br. J. Cancer 84:796-801.[PubMed]).
Detection of high-risk HPV DNA at 6 months after treatment was more sensitive than abnormal cytology findings in patients with moderate or severe cervical disease prior to treatment. The negative predictive value of absence of high-risk HPV DNA and normal cytologic test results in these patients was 99%.
The utility of HPV DNA testing for residual disease following treatment of lower grades of dysplasia remains to be evaluated.
Microinvasive cancers less than 3 mm in size are managed conservatively by excisional cone biopsy.
Early invasive cancers are managed with radical hysterectomy or external-beam high-energy (to 18 MV) radiotherapy and implants loaded with 192Ir. The goal of this therapy is to destroy malignant cells in the cervix, paracervical tissues, and regional lymph nodes. Selected patients also benefit from concurrent chemotherapy.
Locally advanced cancers are managed with radiotherapy to the primary tumor and potential sites of regional spread.
In addition to surgical and cytodestructive procedures, several antiviral and immunomodulatory agents have been evaluated as treatment for HPV-associated cervical lesions. Cidofovir is an acyclic nucleoside phosphonate derivative which has broad-spectrum activity against DNA viruses and is in use clinically for the treatment of CMV infections. Exposure of human carcinoma cell lines containing HPV-16 or HPV-18 and human cervical keratinocytes immortalized by HPV-33 to cidofovir resulted in inhibition of cell proliferation (9 Andrei, G., R. Snoeck, J. Piette, P. Delvenne, and E. DeClercq. 1998. Antiproliferative effects of acyclic nucleoside phosphonates on human papillomavirus (HPV)-harboring cell lines compared with HPV-negative cell lines. Oncol. Res. 10:523-531. [PubMed]).
The in vitro antiproliferative activity was shown to be selective for the rapidly proliferating HPV-infected cells when normal primary human cervical keratinocytes were treated similarly. A 1% cidofovir gel was used topically without side effects every other day for 1 month to treat 15 women with severe CIN (10 Snoeck, R., J. C. Noel, C. Muller, E. De Clercq, and M. Bossens. 2000. Cidofovir, a new approach for the treatment of cervix intraepithelial neoplasia grade III (CIN III). J. Med. Virol. 60:205-209. [PubMed]). Complete or partial response was seen in 80% of patients as assessed by histology and detection of HPV DNA by PCR.
Podophyllin, a cytotoxic agent that arrests mitosis in metaphase (also used to treat genital warts), in combination with vidarabine, a DNA polymerase inhibitor, suppressed HPV gene expression and cell growth in cervical cancer cell lines (11 Okamoto, A., C. D. Woodworth, K. Yen, J. Chung, S. Isonishi, T. Nikaido, T. Kiyokawa, H. Seo, Y. Kitahara, K. Ochiai, and T. Tanaka. 1999. Combination therapy with podophyllin and vidarabine for human papillomavirus positive cervical intraepithelial neoplasia. Oncol. Rep. 6:269-276. [PubMed]).
The expression of HPV-16 E6 and E7 gene products in normal cervical keratinocytes in vitro in the presence of either podophyllin or vidarabine sensitized these cells to apoptosis. Combined topical therapy with podophyllin and vidarabine ointments in 28 patients with mild to moderate CIN resulted in regression of lesions and successful eradication of HPV-16 or HPV-18 DNA in 81% of patients.
The IFNs and intravaginal 5-fluorouracil have shown variable response in clinical and in vitro studies.
IFN-α is approved for treatment of genital warts. The effects of IFN-α, IFN-β, and IFN-γ in several human carcinoma cell lines containing HPV-16 or HPV-18 have been studied (12 Kim, K. Y., L. Blatt, and M. W. Taylor. 2000. The effects of interferon on the expression of human papillomavirus oncogenes. J. Gen. Virol. 81:695-700. [PubMed]).
Response was seen in some cell lines but not others. In HPV-18 HeLa cells, all IFNs suppressed the levels of HPV E6 and E7 gene transcripts. In HPV-18 C-411 cells, IFNs had no effect. In HPV-16 CaSki and HPK1A cells, only IFN-γ was effective. It is likely that, since IFN-responsive elements appear to be down-regulated by at least some oncogenic HPV types, the utility of IFN therapy in cervical disease will be limited (13 Chang, Y. E., and L. A. Laimins. 2000. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J. Virol. 74:4174-4182. [PubMed]).
PREVENTION
Primary approaches to prevent HPV infection include both risk reduction and development of HPV vaccines.
Use of latex condoms and a spermicide may decrease the risk of contracting HPV. Condoms, however, are not totally reliable, since HPV may be contracted by contact with other parts of the body, such as the labia, scrotum, or anus, that are not protected by a condom.
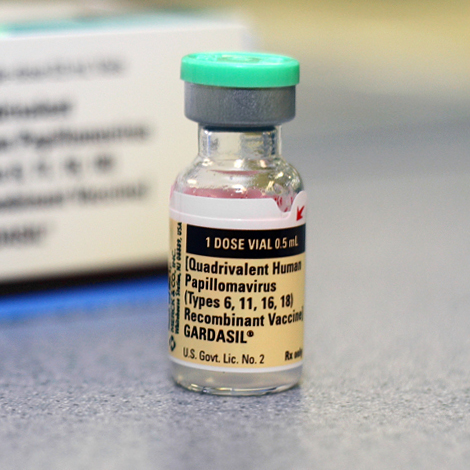
Vaccines directed against HPV are in phase I and phase II clinical trials but are not currently commercially available. HPV vaccines are usually composed of virus-like particles (VLPs), which are empty virus capsids containing the major HPV capsid antigen and possibly the minor capsid antigen but lacking viral DNA. The vaccines are produced by expressing the L1 or L1 and L2 ORFs in eukaryotic cells. These proteins then self-assemble into VLPs which are highly immunogenic. Because of the high level of antigenic specificity of HPV capsid antigens, there is no cross-protection among subtypes, and protection against each subtype requires vaccination with VLPs of that subtype. Optimal vaccines would contain a cocktail of VLPs of the most common high-risk HPV subtypes. Preliminary reports indicate that animal papillomavirus vaccines have excellent immunogenicity and protection against experimental animal papillomavirus diseases.
A double-blind, randomized, placebo-controlled phase I safety and immunogenicity trial has been conducted using a subunit vaccine composed of VLP formed from the entire L1 major capsid protein of HPV-16 strain 114K (14 Harro, C. D., Y.-Y. S. Pang, R. B. S. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [PubMed]). The vaccine was prepared by inserting the L1 capsid gene into a baculovirus vector. The gene was then expressed in transfected Sf9 insect cells. An optimal dose of 50 μg of HPV-16 L1 VLP vaccine was administered by injection into the deltoid muscle at 0, 1, and 4 months. The vaccine generated high titers of type-specific neutralizing antibodies without adjuvant and was well tolerated.

SCREENING STRATEGIES
Screening for cervical cancer remains an important health and economic concern in the United States and throughout the world.
Many studies over several decades have helped elucidate the natural history and pathogenesis of cervical neoplasia.
The incidence of cervical cancer and its associated mortality have declined in recent years, largely due to the widespread implementation of screening programs that use Pap smear testing for detection of abnormal cervical cells.
Methods such as fluid-based cytology have recently been developed that improve the ability to detect precursor lesions in Pap smears and allow detection at an earlier stage.
The Bethesda System reporting of Pap smear results has also recently been updated to improve the utility and understandability of results.
Molecular and epidemiologic studies have solidified the association between high-risk strains of HPV and cervical squamous cell carcinoma.
Tests have been developed to detect high-risk HPV DNA with high sensitivity and specificity in cervical samples. These tests are now available in clinical laboratories at many medical centers and in reference laboratories.
Large-scale studies such as the National Cancer Institute ALTS trial to evaluate management options for women with abnormal Pap smear results have been completed. Particularly problematic are women with ASC-US borderline or equivocal Pap smear results. These studies indicate the potential utility of HPV DNA testing in the management of women with ASC-US Pap smear results. Based on the results of these studies, new screening strategy options that include testing for high-risk HPV DNA as an adjunct to cytology have been developed to triage and monitor ASC-US patients. These strategies are meant to minimize unnecessary follow-up visits and invasive procedures without compromising the detection of disease.
The improvements in cytologic screening as well as the introduction of HPV DNA testing greatly facilitate the identification of women at risk for cervical cancer. Early identification and intervention will probably have a significant impact on the reduction of cervical cancer morbidity and mortality.
Modifications to screening strategies will also probably be made in the future. Retrospective data analysis indicate that it may be advantageous to lengthen the Pap smear screening interval from annually to every 2 to 3 years.
Results from ongoing studies will help clarify the benefits and risks of lengthened screening intervals. Several other studies to examine the role of HPV DNA testing as a primary screening method for cervical cancer are under way. In addition to changes in screening strategies, effective therapeutic and preventive vaccines may be developed that have the potential to contribute significantly to the control and prevention of cervical cancer.
