DEFINITION
In the context of Toxicology, arsenic (the 33rd element of the Periodic Table) is a metalloid that above certain quantities or periods of exposure can cause a pathological effect on human health (arsenicosis). It is present in air, water and soil, so human exposure is either through oral route (food and water) or through inhalation (exposure to agricultural pesticides and mining activities).
Among all forms of chronic arsenic toxicity, the major derives from contamination of drinking water from natural geological sources rather from mining, smelting or agricultural sources. The WHO guidelines describe safety limit of arsenic at 10 µg/L (10 ppb) and a maximum permissible limit of 50 µg/L (50 ppb) of drinking water. If these limits are not respected, people that are being exposed risk developping a severe form of arsenicosis with skin lesions, cancer and various diseases affecting all body systems.
EPIDEMIOLOGY
Over 200 million people are at risk of chronic arsenicosis worlwide, out of which more than half are residing in Bengal Delta Plain including Bangladesh and the State of of West Bengal (India). The accumulation of arsenic in this region is mainly due to geological reasons, and the high prevalence of arsenicosis is due to the fact that the local population do not always have access to safe tube wells.
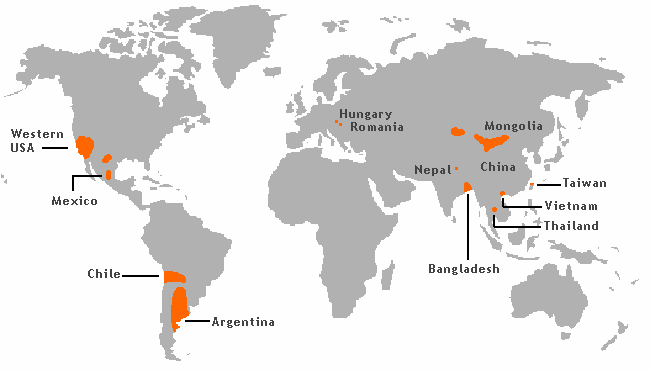
Other areas at risk include Eastern Asia (China, Taiwan, Thailand), South America (Argentina, Chile) and some locations in the United States.
In Italy, in 2010, 128 municipalities in 5 regions (Lazio, Toscana, Trentino, Lombardia, Umbria) were declared as not fulfilling the criteria of safe drinking water imposed by the European Union.
Some medical doctors suggest a minimum time gap of five years between first exposure to contaminated water and the initial symptoms of chronic arsenicosis.
The most evident non-cancer effect of the disease are skin lesions, such as hyperpigmentation (dark spots on the skin) and hypopigmentation (white spots on the skin). These forms of melanosis tipically appear in a raindrop pattern on the trunk or extremities against a tan-to-brown hyperpigmented background. Over time, arsenic exposure is associated with hyperkeratosis, that appears predominantly on the palms and the plantar aspect of the feet.

Other symptoms of chronic arsenic exposure concern:
1. the gastrointestinal system (hepatomegaly, nausea, anorexia, abdominal pain, diarrhoea);
2. the nervous system (weakness, paresthesia, peripheral neuropathy);
3. the respiratory system (cough, dyspnoea);
4. the cardiocirculatory system (e.g. the Blackfoot Disease, endemic in Taiwan, consists in a severe systemic arteriosclerosis with ischemia, dry gangrene and spontaneous amputations of affected extremities).
Arsenic is a pernicious environmental carcinogen and leads mainly to cancers of the skin: these lesions are frequently multiple and involve covered areas of the body, contrary to non-arsenical skin cancer which usually presents as a single skin lesion in exposed parts of the body. However, arsenic may have carcinogenic effects also on lung, bladder, liver and kidney.
The symptoms that may help the medical doctor in the diagnosis of chronic arsenic exposure are the characteristic dermatological features and a history of at least six-month exposure to arsenic levels greater than 50 µg/L or exposure of high arsenic level from food and air. However, most of the factors mentioned in the "Symptoms" paragraph do not have a diagnostic value as they are non specific and can occur with unrelated medical conditions.
In this context, some laboratory exams may be useful:
1. Urinary excretion. The concentration of total arsenic in urine has often been used in laboratory because urine is the main route of excretion of most arsenic species. The half time of inorganic arsenic in humans is about 4 days. In Europe, average background arsenic concentration in urine are generally below 10µg/L; concentrations above this limit may indicate arsenic exposure. However, this exam needs to be evaluated carefully as seafood in the diet may influence urinary arsenic measurements (fish, crustaceans and molluscs contain organic arsenic compounds, which are not toxic for mammalians) and the solutes' concentration in the urine may vary along with the urine osmolality. In order to correct these distortions, some clinical laboratories use a speciation method that only measures inorganic arsenic or its metabolites related to the concentration of creatinine or the specific gravity.
2. Hair and nail. Arsenic tends to concentrate in these tissues because of their high content of keratin. In people with no known exposure to arsenic the concentration of arsenic in hair is generally 0.02-0.2 mg/kg; patients with chronic arsenic poisoning may have hair concentrations varying from 10 ppm (10 mg/kg) to 100 ppm. Normal arsenic values in nails appear to range from 0.02 to 0.5 mg/kg; several tens of mg per kg have been repoted in cases of chronic poisoning. However, even these tests need to be interpreted appropriately as they can be distorted by external arsenic contamination.
3. Blood. Data on concentrations of arsenic in blood in people with no known exposure to arsenic are in the range of 0.3-2 µg/L; in people exposed to arsenic in drinking water, the mean blood concentration can reach 10 µg/L. This test is much less sensitive than the urine exam because of the analytical error and because arsenic in blood is cleared fairly rapidly in man. Nonetheless, the blood exam can be used for the diagnosis of anemia, which is common in patients suffering from arsenicosis.
The most common oxidation numbers of arsenic are +5, +3 and -3, in which the element is able to form both inorganic and organic compounds both in the environment and within the human body. However, only the inorganic forms seem to be dangerous for the human health: arsenic trioxide is the most prevalent inorganic arsenical found in air, while a variety of inorganic arsenates or arsenites occur in water, soil or food. Arsenite, which is more toxic than arsenate, is the preferential form of introduction of arsenic in the human body.
The major site of absorption is the small intestine by an electrogenic process involving a proton gradient; the optimal pH for arsenic absorption is 5, though in the small bowel the pH is approximately 7.
The metabolism of inorganic arsenic involves a two-electron reduction of pentavalent arsenic to trivalent arsenic, mediated by glutathione, followed by oxidative methylation to form pentavalent organic arsenic. Thus, the metabolites of arsenic after hepatic biomethylation are MMA (monomethylarsonic acid) and DMA (dimethylarsonic acid) that are less toxic but not completely innocuous. DMA is the dominant urinary metabolite (60-70%) compared with MMA; a small amount of inorganic arsenic is also excreted unchanged. The methylation of inorganic arsenic is considered to be a detoxification mechanism, but MMA and DMA are still carcinogenic and the liver rapidly becomes unable to cope with the large entering amount of arsenite, that tends to be stored in many organs. In these tissues, arsenic induces morphologic changes in mitochondrial integrity and a rapid decline of mitochondrial membrane potential; these alteration lead to an uncontrolled random formation of superoxide anion radical (O2-) and oxidative stress. The formation of reactive oxygen species (ROS), along with the ability of arsenic to inactivate up to 200 cellular enzymes (especially those involved in cellular energy pathways and DNA synthesis and repair), seems to be one of the main mechanisms responsible for its toxicity.
1. Arsenic-induced nephrotoxicity and hepatotoxicity. The oxidative stress causes an up-regulation of HO-1 (hemeoxygenase-1) and MAPK, which cause the activation of transcriptional factors such as AP-1, ATF-2, Elk-1 with consequent nephrotoxicity. The ROS cause hepatotoxicity by stimulating lipid peroxidation, reducing the activity of antioxidant enzymes and up-regulating pro-apoptotic proteins such as JNK and p38MAPK.
2. Arsenic-induced diabetes mellitus. Arsenic causes decreased expression of PPAR-γ, which reduces the sensitivity of insulin. Moreover, arsenic replaces a phosphate group from ATP forming ADP-arsenate which slows down the metabolism of glucose. The ROS cause overexpression of various stress mediators such as NF-KB and JNK-SAPK with β cell dysfunction and insulin resistance. Finally, arsenic decreases the activity of PDK-1 with inhibition of insulin-dependent glucose uptake.
3. Arsenic-induced neurotoxicity. Arsenic crosses freely the blood-brain barrier, causing oxidative stress with consequent oxidative DNA damage and lipid peroxidation. Moreover, it alters the metabolism of various neurotransmittors and causes apoptosis through induction of p38MAPK and JNK. It also causes thiamine deficiency and inhibits pyruvate decarboxylase with consequent accumulation of blood pyruvate and encephalopathy. Arsenic decreases acetyl-cholinesterase activity, diminishes the nerve conduction velocity and suppresses the NMDA receptors in hippocampus.
4. Arsenic-induced cardiovascular dysfunction. The ROS couples with NO to form peroxynitrite, a strong oxidant implicated in the upregulation of inflammatory mediators such as COX-2. The ROS also increase the expression of atherosclerosis related genes such as hemeoxygenase-1 (HO-1) and IL-6 with consequent migration of monocytes. Arsenic decreases the activity of eNOS, mediates vasoconstriction of the blood vessels by phosphorylating MLCK and increases calcium sesnsitization leading to hypertension.
5. Arsenic-induced carcinogenicity. Oxidative DNA damage causes chromosome aberration and interference with cellular signaling. Arsenic also changes the expression of genes that are involved in cell growth, proliferation and malignant transformation.
A high risk factor is living in a geographical area with contaminated drinking water. The extent of arsenic poisoning depends on various factors such as dose, age of the affected individuals and individual susceptibility to arsenic. Genetic polymorphism af the arsenic methylation enzymes may help explain the inter-individual variation, even within the same family; similar genetic differences may exist in arsenic-specific binding proteins, which are thought to decrease the toxicity of inorganic arsenic by decreasing its tissue availability until it can be methylated. Other risk factors may include malnutrition (diet low in proteins and vitamins), which compromises the liver's ability to methylate, and infection with hepatitis B virus.
In chronic arsenicosis, the liver is particularly concerned as it is the first organ to directly receive the mass of arsenic that has been ingested. The inability of the liver to cope with this quantity, as well as the harm to the hepatocytes due to oxidative stress, causes arsenic accumulation in kidneys, heart and lungs and smaller amounts in the muscles, nervous system, gastrointestinal tract and spleen. Though most arsenic is cleared from these sites, residual amounts remain in keratin-rich tissues such as nails, hair and skin. In general, arsenicosis is not a single-organ disease but usually involves many tissues, organs and systems.
COMPLICATIONS
As mentioned above, chronic arsenicosis can lead to different severe diseases involving all organs and systems; among these diseases, we can remember cancer, encephalopathy, peripheral neuropathy, diabetes, arteriosclerosis and pulmonary fibrosis. The lethal dose of arsenic for humans has been estimated to be about 0.6 mg/kg/day.
There is no effective therapy for chronic arsenicosis; patients once affected may not recover even after remediation of the arsenic contaminated water. However, there are treatments for relief of systemic clinical manifestations and reduction of arsenic stores in the body:
1. Chelation therapy. A chelating agent forms a ring structure with the offending metal and increases its excretion by the kidney. The most used chelators are DNSA (dimercaptosuccinic acid), DMPS (dimercaptopropane succinate) and D-penicillamine. However, their usefulness has not entirely been proved and they may have toxic side effects.
2. Retinoids. Oral supplementation with vitamin A seems to cause regression in arsenical keratosis. Moreover, the interaction of retinoids with nuclear receptors influences the expression of genes that affect cell differentiation, proliferation and induction of apoptosis.
3. Antioxidant protection. This therapy, consisting for example in the administration of α-tocopherol and ascorbic acid, may be beneficial against DNA oxidative stress.
4. Supportive treatment. In malnourished patients, high-protein-containing diet possibly helps in clearance of inorganic arsenic (more toxic) by increased methylation. The various clinical manifestations should be treated symptomatically.
PREVENTION
The most important action in affected communities is the prevention of further exposure to arsenic by provision of safe drinking water.
In developed countries at risk small "under the sink" units, which act as filters, have been used to remove arsenic from drinking water. However, in many states all over the world, all the water aupplied to residences by utilities must meet primary health-based drinking water standards: in this context, large-scale water treatment is possible. Among the techniques used in this field, we can mention:
1) Coagulation/filtration/flocculation (coprecipitation and adsorption of arsenic using iron coagulants);
2) Iron oxide adsorption (that fliters the water through a granular medium containing ferric oxide);
3) Activated alumina (an adsorbent that effectively removes arsenic);
4) Ion exchange (it is effective in removing arsenate but not arsenite, which does not have a net charge);
5) Reverse osmosis and electrodyalisis (it can remove arsenic with a net ionic charge);
6) Subterranean arsenic removal (iron and arsenic compounds are rendered inactive in the aquifer itself thanks to arsenic-oxidizing microorganisms which can oxidize arsenic from +3 to +5 state).
In developing countries, other measures can be taken:
1. Deeper wells are often less likely to be contaminated.
2. It may be possible to use treated surface water and rainwater for drinking and contaminated groundwater for other purposes, like laundry;
3. It is important to inform people living in arsenic-contaminated areas about the risks linked to arsenicosis and offer them health and economical support.