Gestational diabetes: the role played by TNFα
Definition

Gestational diabetes, also known as gestational diabetes mellitus ( GDM ), occurs when women, who have never shown previously diagnosed diabetes, exhibit high blood glucose levels during pregnancy. This condition appears frequently in the third trimester. GDM is probably due to the fact that insulin receptors don’t work properly: in fact, there are a lot of factors, associated with pregnancy ( such as “adipocytokines” ), that can interfere with their function, leading to extremely high blood sugar levels.
GDM has few symptoms and it is generally diagnosed by screening during pregnancy. If untreated, it can lead to several risks, not only for the mother, but even for the baby: the former, in fact, may develop type 2 diabetes mellitus or type 1, while the latter might suffer from jaundice, seizures and may put on extra weight. On the other hand, it is known that women with GDM, who try to overcome it recurring to diet or exercise, may have smaller birthweight babies and, consequently, problems such as survival rate of premature and early births.
Classification
GD is defined as follows: “Any degree of glucose intolerance with onset or first recognition during pregnancy”. From Priscilla White derives the White classification, that divides that kind of diabetes into two groups:
• Gestational diabetes, subdivided into:
o TYPE A1: abnormal oral glucose tolerance test (OGTT), but normal blood glucose levels during fasting and two hours after meals;
o TYPE A2: abnormal OGTT compounded by abnormal glucose levels during fasting and/or after meals; in this case, it is needed an additional therapy with insulin;
• Pre gestational diabetes:
o Type B: onset at age 20 or older and duration of less than 10 years.
o Type C: onset at age 10-19 or duration of 10–19 years.
o Type D: onset before age 10 or duration greater than 20 years.
o Type E: overt diabetes mellitus with calcified pelvic vessels.
o Type F: diabetic nephropathy.
o Type R: proliferative retinopathy.
o Type RF: retinopathy and nephropathy.
o Type H: ischemic heart disease.
o Type T: prior kidney transplant.
Screening
( Glucose screening and glucose tolerance tests, October 2011 )
All pregnant women should be screened for gestational diabetes during their pregnancy. Screening can be done by taking the woman's medical history, evaluating certain risk factors, or screening with an oral glucose tolerance test ( Pregnancy and Gestational diabetes screening, July 2012 ), between 24 and 28 weeks of pregnancy. It would be better doing the OGTT in the morning and, during the three previous days, the woman must have at least 150 g carbohydrate/day. The test consists in drinking a solution with an amount of glucose ( 75-100 g ). Moreover, the blood must be analysed at the beginning of the test and after set time intervals. These values are considered abnormal during the OGTT:
• Fasting blood glucose level ≥95 mg/dl (5.33 mmol/L)
• 1 hour blood glucose level ≥180 mg/dl (10 mmol/L)
• 2 hour blood glucose level ≥155 mg/dl (8.6 mmol/L)
• 3 hour blood glucose level ≥140 mg/dl (7.8 mmol/L)
Epidemiology
It has been shown that, during the last 20 years, the percentage of subjects suffering from GDM has increased by 50 % in different Countries ( Increasing prevalence of GDM, July 2007 ). GDM is strictly related to advanced maternal age, obesity and type 2 diabetes. Other important risk factors are:
• Polycystic ovary syndrome
• A previous diagnosis of GD or prediabetes
• Short stature
• Smoke
• Having too much amniotic fluid ( polyhydramnios )
• Previously giving birth to a stillborn child or a baby over 9 pounds
It is important to underline that the prevalence of this disease is not equally distributed in different ethnic groups: for example, with reference to U.S, Asian and Hispanic women are at risk for GDM more than non-Hispanic white ones; instead, in Europe, gestational diabetes is more widespread among Asian women than among European women.
Pathophysiology: TNFα
( Pathophysiology of GDM: the past, the present and the future, November 2011 )
Actually, it is still unknown the precise mechanisms related to GDM. With no doubt, the insulin resistance plays an extremely important role. It is believed that factors and pregnancy hormones interfere with the binding of insulin to its receptors, thus influencing the cellular signaling cascade that follows. Considering that insulin stimulates the uptake of glucose into cells ( by increasing the number of GLUT4 receptors ), the level of this sugar in the bloodstream rises dramatically.
Insulin resistance is a normal phenomenon ( caused by maternal estrogen and progesterone promoting pancreatic β cell hyperplasia ) which occurs during the second trimester of pregnancy with the purpose of ensuring an adequate supply of glucose to the fetus. Placental hormones, as well as fat deposits during pregnancy, increase this phenomenon in women suffering from GDM. Thus, glucose, which is able to cross the placenta by diffusion facilitated, leads to an increase of the production of fetal insulin. For this reason, fetus may be affected by macrosomia, due to the excessive growth promoted by insulin.

As previously said, there are different factors involved in insulin resistance, related to the so called “feto-placeta unit”:
• The placenta synthesizes progesterone and pregnenolone from cholesterol, which are carried to fetal adrenal glands where they are turned into cortisol/corticosterone or into DHEAS. DHEAS are transported back to the placenta where are turned into estrgenes ( estriol is the principal ). Cortisol is considered to be the main hormon involved into the reduction of glucose tolerance during pregnancy, while estrogenes and progesterone are though to be important for insuline resistance in late pregnancy;
• Human placental lactogene ( hPL ), strictly related to fetal and placental weight, mobilizes lipids and free fatty acids, in order to supply a different source of nourishment to the mother, while preserving glucose for the fetus. The increase of free fatty acids reducts the enter of glucose into cells;
• Level of prolactin rises during pregnancy, especially in the third trimester when it is more probable to develop GDM. However, it is not demonstered that prolactin has direct effects on glucose intolerance.
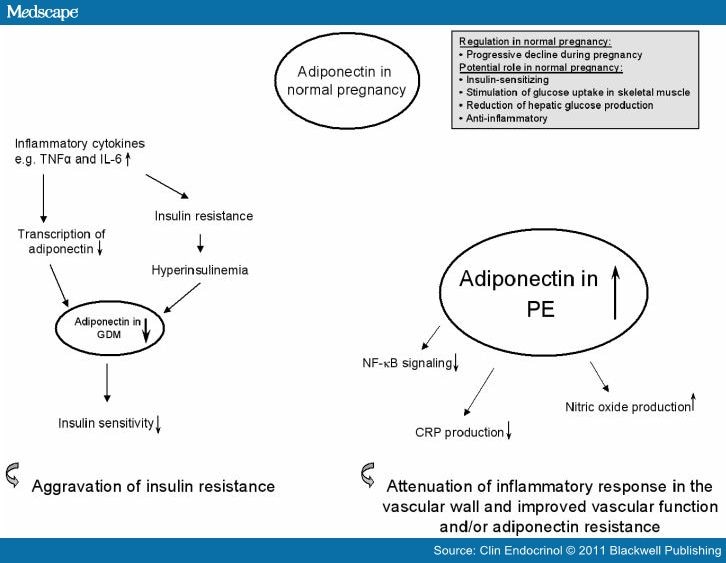
Also adipose tissue is involved in the reduction of glucose tolerance in GDM: it has been shown that this tissue products factors ( “adipocytokines” ), acting as hormones, such as adiponectin, resistin, leptin, IL-6 and tumor necrosis factor α. The latest seems to be the principal marker of GDM.
TNFα
( Diabete gestazionale:un'opportunità per prevenire il diabete di tipo 2 e malattie cardiovascolari nelle giovani donne, January 2010 )
Tumor necrosis factor α, inducing tumor necrosis and released from macrophages, is strictly related to insulin resistance, showing an increse of its level in the bloodstream during the third trimester of pregnancy. It has been demonstered that TNFα mRNA is present in the placenta both at the beginning and at the late stages of pregnancy. Moreover, tumor necrosis factor levels in bloodstream are extremely higher in obese women, as weel as in ones suffering from GDM.
o TNFα is though to interfere with the insulin receptor autophosphorylation, activating a cascade that increase ceramides and sphyngomielinase. It exerts its action in adypocites, skeletal muscle and hepatocytes ( Ceramide mediates TNF-alpha-induced insulin resistance on GLUT4 gene expression in brown adipocytes, 2006 ) ( A Role for Sphingolipids in Producing the Common Features of Type 2 Diabetes, Metabolic Syndrome X, and Cushing's Syndrome, 2005 ); It has been shown, in fact, that these factors may inhibit both IRS-1 and Akt/PKB;

o What is more, it reducts the association between IRS-1 and IR by the serine phosphorylation of IRS-1 ( this can be seen especially in sketal muscle in late gestation ) (Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways, January 2001 ). It is pretty sure that the involved serine is the 307th;
o In women suffering from GDM, the entrance of glucose in skeletal muscle is significally decreased, even if the numebr of GLUT4 carriers is normal; these transportes are reduced in adipose tissue, instead ( Glucose Transporters and Pathophysiologic States from the book titled Diabetes Mellitus: A Fundamental and Clinical Text, 2004 );
o TNFα suppresses the espression of PPARγ, which is thought to increase the level of adiponectin, both in normal and GDM subjects. TNF alpha is able to reduce PPARgamma transcriptional activity by serine phosphorylation, due to JNK or ERK1/2. Even NFkb, promoted by the TNF, leads to this result. ( TNF-α and adipocyte biology, 2007 ) ( Negative regulation of peroxisome proliferator-activated receptor-gamma gene expression contributes to the antiadipogenic effects of tumor necrosis factor-alpha, 1996 ) ( REGULATION OF PPARγ FUNCTION BY TNF-α, July 2008 );
o It reduces also the espression of adiponectin gene: this factor is considered to be anti-diabetc, ameliorating insuline resistance and being negatively correlated to obesity ( if adiponectin level falls down, it can been seen an increase in body fat mass ) ( Relationship between plasma adiponectin and TNFalpha concentration in women with gestational diabetes, 2006":http://europepmc.org/abstract/CBA/629947 );
( Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes, July 2005 )
Therapy
First of all, it is essential to monitor the blood sugar of women suffering from GDM even 4/5 times a day. What is more, it is important to follow a healthy diet, including fruits, vegetables and whole grains. Pregnant subjects should practise regular physical activity, which stimulates the entrance of glucose into cells where it is used for energy, as well as increases insulin sensitivity. If these are not enough, insulin injections may be needed to lower the percentage of glucose in the bloodstream. Glyburide, an oral drug, may be prescribed to keep blood sugar under control.