Introduction
D-limonene (1-methyl-4-(1-methylethenyl)cyclohexane) (molecular formula: C10H16 ) is a common cyclic terpene in nature. It is contained in the oil of several citrus fruits such as oranges, mandarins, grapefruit and lemons, from which it takes its name.


D-limonene is synthetized from geranyl pyrophosphate (an intermediate in the HMG-CoA reductase pathway) through a reaction of cyclization.

Being a quite stable chemical compound it can be extracted by steam distillation of citrus peels and pulp or from deterpenation of citrus oil. Because of its pleasant fragrance and lemon taste it is widely used by the industry as a flavor and fragrance additive in several products (perfumes, foods, beverages). Moreover it is used in pesticides and also as a natural replacement of petroleum-based solvents.
Dietary intake of D-limonene may vary considerably depending on the amount of citrus consumed and possible processing procedures. The daily per capita consumption in the USA has been estimated in 16.2 mg/day. We may speculate that in the Mediterranean diet the intake might be a little higher.
Clinical studies report its activity in dissolving human gallstones while administered IV and in relieving heartburn and gastroesofageal reflux disorder.
In vitro studies well established D-limonene anti-inflammatory and anticancer effects on cell lines. A phase 1 clinical trial in 1998 acknowledged the compound well tolerability in patients with advanced solid cancers even if possible clinical benefits were not studied.
Nevertheless further investigation is still needed to assess D-limonene possible role as an effective cancer chemopreventive or therapeutic agent in human beings.
Limonene
Pharmacokinetics
D-limonene pharmacokinetics has been studied by making voluntaries drink 30 to 40 OZ (887 to 1182 milliliters) of lemonade (containing 447 to 596 mg of D-limonene) and by taking blood sample every hour for 24 hours. The distribution of D-limonene and its metabolites has been demonstrated on several animal models such as rats, rabbits, hamsters and pigs using radioactively marked D-limonene.
Orally administrated D-limonene is rapidly absorbed in the GI tract and it is quickly distributed to several tissues.In peripheral tissues the compound is rapidly metabolized by monoxygenases, probably belonging to the cytochrome P450 family, to oxidized metabolites such as perillic acid, dihydroperillic acid, limonene-1,2diol and uroterpenol. These compounds are particularly detectable in liver, lung, serum, kidney, mammary gland and adipose tissue.
Plasma levels of D-limonene and perillic acid (its main metabolite) peak after 1 h since oral administration and rapidly decrease with an half-life of about 1.38 h (0.82-1.84 h). 24 h after administration the compound levels are undetectable. The maximum plasma concentration of such molecules is reported to vary from 2.08 to 13.9 µM, depending on the subject.
D-limonene and its metabolites are mainly conjugated with glucoronic acid and subsequently excreted with the urine.
Phase I pharmacokinetic trial of perillyl alcohol (NSC 641066) in patients with refractory solid malignancies. 2000
Pharmacokinetics of Perillic Acid in Humans after a Single Dose Administration of a Citrus Preparation Rich in d-Limonene. 2002
Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. 1998
Studies on the metabolism of d-limonene (p-mentha-1,8-diene). IV. Isolation and characterization of new metabolites and species differences in metabolism. 1976
Toxicity and side effects
D-limonene has been reported to have both toxic and carcinogenic effects on rats, causing body weight loss, lethargy, nephropathies and also tubular renal cancer. The mechanism responsible for murine nephropathies and renal cancers seems to be connected with the accumulation of hyaline-droplets in proximal tubules. Such droplets consist mainly of α2u-globulin (α2u-g, a rat-specific low molecular weight protein) bonded with D-limonene. There’s no possibility of such reaction in humans because α2u-globulin is not present in human kidney tissue.
A phase I pharmacokinetic study demonstrated no toxicity on cancer patients with an MTD (maximum tolerated dose) of 8g/m2/day thus establishing D-limonene to have no toxic or carcinogenic effects on humans. Moreover genotoxicity and mutagenic tests for D-limonene on human cells are reported to be negative.
Clinical knowledge report modest side effects even at high dosages:
* nausea
* vomiting
* diarrhea
Such effects are strictly dose-dependent. Also long-term possible toxicity has been investigated and even at MTD for 11 months D-limonene appears to be safe and well tolerated.
We can assert that D-limonene is safe on humans at it causes only little side effects at pharmacological concentrations, far from those obtainable with a normal dietary intake of such compound.
d-limonene mechanistic data and risk assessment: absolute species specific cytotoxicity, enhanced cell proliferation, and tumor promotion. 1996
D-Limonene: Safety and Clinical Applications. 2007
Pharmacodynamics
D-limonene and its metabolites have two different and clearly established effects on cell lines:
* anti-inflammatory
* pro-apoptotic on cancer cells
The anti-inflammatory activity has been studied on macrophages exposed to LPS (Lipopolysaccharide, the endotoxin present on Gram-negative bacteria outer cell membrane), a molecule which triggers a powerful immune response.
The interaction of LPS with the TLR-4/CD-14 receptor complex generally activate the innate immune response mediated by macrophages. The extracellular domains of the TLR binds the LPS while the intracellular domains activate a pathway involving several proteins in precise sequence: MyD88 and MAL, IRAK1 and IRAK4, TRAF6, TAK1. TAK1 has a double effect, activating two different pathways: the MAPK signaling pathway and the IkB kinase. The former has a mitotic effect, allowing immune cells proliferation; the latter permits the migration of the transcription factor NF-kB into the nucleus. Interacting with DNA NF-kB modifies the cell profile of gene expression promoting the transcription of several genes codifying for inflammatory mediators such as COX-2, iNOS, IL-1, IL-6 and TNFα.
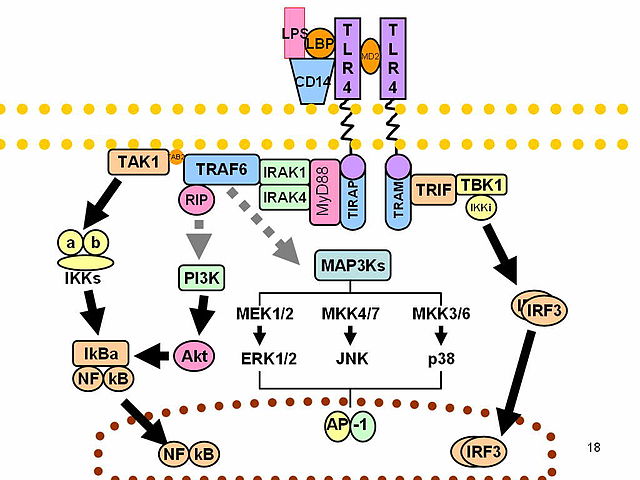
Macrophages treated with D-limonene, despite being exposed to LPS, produce inflammatory mediators in much lower concentrations compared to controls while their viability is not modified. The inhibition of such proteins shows to be linearly connected with the concentration of D-limonene. The inhibition of the NF-kB signaling seems to have an important role in this phenomenon however, to date, it is impossible to exclude that D-limonene affects the activity of other transcription factors in immunity cells.
The second and maybe most important activity and D-limonene and its metabolites is their effect on cell cycle and viability in several cancer cell lines. It has been demonstrated that D-limonene and its metabolites have the effect of down regulating the expression of Bcl-2, an important regulator of the mitochondrial pathway of apoptosis which prevent the formation of the apoptotic pores mediated by the BAX protein. Bcl-2 is often overexpressed in cancer cells. The down regulation of Bcl-2 and the contemporary up regulation of BAX lead to the release of cytochrome c in the cytosol thus activating the apoptotic mechanism regulated by caspase-9 and caspase-3. This series of events results in apoptosis.
Recent data report another possible mechanism which permits D-limonene to induce the mitochondrial death pathway. The compound is reported to down regulate the PI3K/Akt/mTOR pathway (often over activated in cancer, allowing proliferation and preventing apoptosis) thus leading to the activation of BAD (Bcl-2 Associated Death promoter), a protein that promotes apoptosis and blocks anti apoptotic proteins by binding them. The inhibition of the PI3K/Akt/mTOR pathway by D-limonene is reported to be dose dependent. This mechanism of action has been reported to occur in different types of cancer, in particular on leukemia cell, on colon cancer cells and even on breast cancer cells.
More data is needed to assess D-limonene effects and possible clinical benefits on cancer patients.
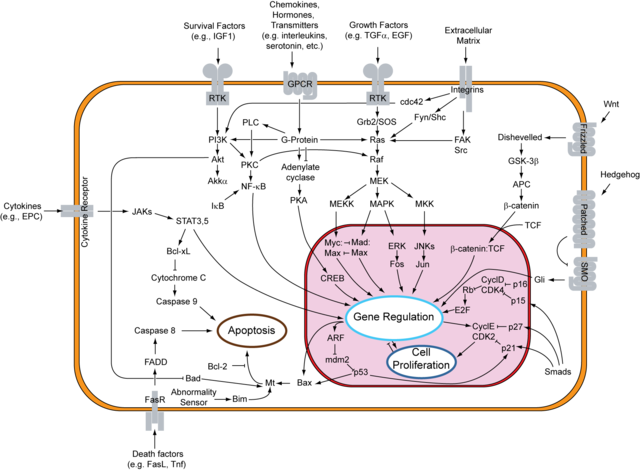
The above reported phenomena are mainly due to the effects of D-limonene and related compounds on the activation of the RAS signaling network.
The processing of RAS protein requires a final farnesylation or geranylgeranylation at a cysteine residue at the fourth amino acid position from the COOH-terminal (sequence CaaX, in which C is cysteine, a is an aliphatic amino acid and X is variable amino acid) to obtain a functional protein. Mutations of that cysteine residue block RAS activity. Such reaction of farnesylation or geranylgeranylation is done by two enzymes: farnesyltransferase (FT) and geranylgeranyltransferase 1 (GGT1). Both enzymes share a functional subunit called FNTα, such protein is cleaved by caspase-3 during apoptosis thus suggesting that some kind of signal may induce apoptosis by indirectly inhibiting the prenylation of proteins involved in cell survival and proliferation.
D-limonene and perillic acid have been reported to be inhibitors of FT and GGT1 thus preventing the prenylation and subsequent activation of RAS and its related signaling cascades. A research group at St. Jude Children's Research Hospital in Memphis, USA, is working about the hypothesis that such compounds occupy an hydrophobic pocket of the prenyltransferases enzymes consequently blocking their activity. In this way these chemicals interfere with the activation of RAS signaling.
Moreover D-limonene and perillic acid are reported to affect the mevalonate pathway in different ways, hence interfering with the production of farnesyl-PP and geranylgeranyl-PP and consequently, with the production of prenylated proteins (not only RAS). In fact recent data proposed that perillic acid and also D-limonene have the ability to modulate gene expression, translational efficiency and also post-translational processing of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA reductase). There is evidence of a reduction of HMG-CoA reductase mass and activity in cells exposed to perillic acid and D-limonene.

The prevailing activity of such compounds seems to be the inhibition of FT and GGT1, thus interfering with the RAS signaling network, which affects not only the cell survival and proliferation but also interacts with the PI3K/Akt pathway and consequently with the NF-kB signaling.
The reduction of RAS prenylation could be an unifying explanation for both the anti-inflammatory and anticancer effects of terpenes contained in citrus fruits.

Effects of D-limonene on leukemia cells HL-60 and K562 in vitro. 2006
D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. 2012
Induction of apoptosis by d-limonene is mediated by a caspase dependent mitochondrial death pathway in human leukemia cells. 2006
Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. 2013
Limonene suppresses lipopolysaccharide-induced production of nitric oxide, prostaglandin E2, and pro-inflammatory cytokines in RAW 264.7 macrophages. 2010
KEGG: NF-kB signaling pathway
Targeting protein prenylation for cancer therapy. 2011
Plant-Derived Monoterpenes Suppress Hamster Kidney Cell 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Synthesis at the Post-Transcriptional Level. 2003
Prenyl-binding domains: potential targets for Ras inhibitors and anti-cancer drugs. 2004
Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2). 1994
Inhibiting Ras prenylation increases the radiosensitivity of human tumor cell lines with activating mutations of ras oncogenes. 1998
Chemoprevention of hepatocarcinogenesis with dietary isoprenic derivatives: cellular and molecular aspects. 2012
Inhibition of farnesyl protein transferase and P21ras membrane association by d-limonene in human pancreas tumor cells in vitro. 1999
Inhibition of Ras prenylation: a novel approach to cancer chemotherapy. 1997
Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. 2004
ANTI-TUMOR MECHANISM OF THE MONOTERPENS (NIH). 2003
Epidemiological evidences
This chemical compound has interesting features, needing further investigation, in order to evaluate its possible role in cancer chemoprevention and, maybe, cure. An epidemiological study of the Arizona Cancer Center has demonstrated that a population with a regular consumption of citrus juices and citrus peels had a lower incidence of squamous cell carcinoma of the skin compared to the US general population.

Citrus peel use is associated with reduced risk of squamous cell carcinoma of the skin. 2000
Conclusion
Provided that one of the mainstream theories about carcinogenesis postulates that chronic and prolonged inflammation has an important role in the development of several kinds of cancer and that subclinical chronic inflammation is an important pathogenic factor in the development of metabolic syndrome, a cluster of common pathologies, including cardiovascular diseases, we may speculate that the anti-inflammatory action of D-limonene and compounds derived from it present in citrus fruits might have an active role in preventing several pathologies, not only cancer.
If such action were confirmed by additional studies we may guess that public health policies would advise people about introducing a certain amount of citrus fruits in their usual diet because of its chemo preventive actions, thus reducing the potential disease burden in an inexpensive way.
The pro-apoptotic properties of D-limonene and its metabolites are certainly very interesting in the oncologic field. However further studies, for example double-armed placebo-controlled trial, might offer an insight about a possible role of such chemical in cancer therapy. An inexpensive and extremely well tolerated compound offering clinical benefits to cancer patients would certainly be an interesting and useful advance in cancer therapy.
We may conclude that the regular consumption of foods containing D-limonene is an healthy and advisable habitude which should be promoted in order to reduce the incidence of several diseases in a simple and quite inexpensive way.