Fetal alcohol syndrome(FAS)

Description
Fetal alcohol syndrome is a pattern of mental and physical defects that can develop in a
fetus in association with high levels of alcohol consumption during pregnancy.
Alcohol crosses the placental barrier and causes various effects in developing brain cells, and this can create an array of primary cognitive and functional disabilities (including poor memory, attention deficits, impulsive behavior, and poor cause-effect reasoning) as well as secondary disabilities (predispositions to mental health problems and drug addiction).
Alcohol exposure presents a risk of fetal brain damage at any point during a pregnancy, since brain development is ongoing throughout the whole pregnancy. It was widely accepted that maternal drinking during pregnancy was one of the strongest predictors of later neurodevelopmental deficits in children with alcohol-induced damage (Neurobehavioral effects of prenatal alcohol: Part III. PLS analyses of neuropsychologic tests, 1989).
However this does not seem to explain FAS entirely, as ethanol alone may not explain the entire spectrum of anomalies seen in individuals living under the poverty level, who are at risk for micronutrient deficiencies due to insufficient intake. In particular, three nutrients commonly found to be deficient are folate, choline and vitamin A. FAS. It is hypothesized that FAS may be caused more by the nutritional deficiencies that are exacerbated by alcohol than by direct alcoholic neurotoxicity. (Proceedings of the 2008 annual meeting of the Fetal Alcohol Spectrum Disorders Study Group, 2009). Thus, supplementation of folate, choline and vitamin A to mothers may mitigate the effects of the alcohol and reduce the severity or prevalence of FAS.
Epidemiology
Fetal alcohol exposure is the leading known cause of mental retardation in the Western world. In the United States and Europe, the FAS prevalence rate is estimated to be between 0.2-2 in every 1000 live births. The lifetime medical and social costs are estimated to be as high as US$800,000 per child born with the disorder. African Americans and Native Americans of low socioeconomic status were shown 10 times more likely to have children with FAS than Caucasian children of middle and upper socioeconomic status. (An update on incidence of FAS: FAS is not an equal opportunity birth defect, 1995)
Signs and symptoms
Growth deficiency
Growth deficiency is defined as below average height, weight or both due to prenatal alcohol exposure, and can be assessed at any point in the lifespan although birth height and weight are the preferred measurements.
Growth deficiency is ranked as follows by the "4-Digit Diagnostic Code”:
• Severe — Height and weight at or below the 3rd percentile.
• Moderate — Either height or weight at or below the 3rd percentile, but not both.
• Mild — Both height and weight between the 3rd and 10th percentiles.
• None — Height and weight both above the 10th percentile.
Facial features
Several characteristic craniofacial abnormalities are often visible in individuals with FAS, indicating brain damage, though brain damage may also exist in their absence. FAS facial features (and most other visible, but non-diagnostic, deformities) are believed to be caused mainly during the 10th and 20th week of gestation.
The three FAS facial features are:
- A smooth philtrum — The divot or groove between the nose and upper lip flattens with increased prenatal alcohol exposure.
- Thin vermilion — The upper lip thins with increased prenatal alcohol exposure.
- Small palpebral fissures — Eye width decreases with increased prenatal alcohol exposure.
Ranking FAS facial features is complicated because the three separate facial features can be affected independently by prenatal alcohol.

Central nervous system
Central nervous system (CNS) damage is the primary feature of any Fetal Alcohol Spectrum Disorder (FASD) diagnosis. Prenatal exposure to alcohol — which is classified as a teratogen — can damage the brain across a continuum of gross to subtle impairments.
The "4-Digit Diagnostic Code" elaborates the degree of CNS damage according to four ranks:
- Definite — Structural impairments or neurological impairments for FAS or static encephalopathy.
- Probable — Significant dysfunction of two standard deviations or worse in three or more functional domains.
- Possible — Mild to moderate dysfunction of two standard deviations or worse in one or two functional domains or by judgment of the clinical evaluation team that CNS damage cannot be dismissed.
- Unlikely — No evidence of CNS damage.
Structural
Structural abnormalities of the brain are observable, physical damage to the brain or brain structures that may include microcephaly (small head size) or other abnormalities (e.g., agenesis of the corpus callosum, cerebellar hypoplasia).
Because imaging procedures are expensive and relatively inaccessible to most patients in the U.S.A., diagnosis of FAS is not frequently made via structural impairments, except for microcephaly.
Neurological
Neurological impairments are caused by prenatal alcohol exposure which causes general neurological damage to the central nervous system (CNS) and the peripheral nervous system (PNS).
Functional
Functional impairments are deficits, problems, delays, or abnormalities due to prenatal alcohol exposure (rather than hereditary causes or postnatal insults often referred to as developmental disabilities.
Alcohol metabolism
Unlike food which requires digestion, the tiny ethanol molecules can diffuse right through the walls of an empty stomach. After absorption, it will collect in the liver and get processed there.
Processes
The first three steps of the reaction pathways lead from ethanol to acetaldehyde to acetic acid to acetyl-CoA. Once acetyl-CoA is formed, it is free to enter directly into the citric acid cycle.
- Ethanol is oxidized to acetaldehyde via the enzyme alcohol dehydrogenase IB (class I). Members of this enzyme family metabolize a wide variety of substrates, including retinol, hydroxysteroids, and lipid peroxidation products.
- The enzyme associated with the transformation from acetaldehyde to acetic acid is aldehyde dehydrogenase 2 family (ALDH2). Two major liver isoforms of aldehyde dehydrogenase, cytosolic and mitochondrial, can be distinguished. Most Caucasians have them both, while approximately 50% of Orientals have the cytosolic isozyme but not the mitochondrial isozyme. The increased exposure to acetaldehyde in individuals with the catalytically inactive form may confer greater susceptibility to many types of cancer.
- Acetaldehyde is a highly unstable compound and quickly forms free radical structures which are highly toxic if not quenched by antioxidants such as ascorbic acid (Vitamin C) and Vitamin B1 (thiamine). These free radicals can result in damage to embryonic neural crest cells and can lead to severe birth defects.
- The enzyme associated with the conversion of acetic acid to acetyl-CoA is ACSS2. The protein acts as a monomer and produces acetyl-CoA in a reaction that requires ATP. Expression of this gene is regulated by sterol regulatory element-binding proteins.

Main consequences of alcohol metabolism
Apart from his role as a source of energy for the cell, alcohol also has different effects on the whole body homeostasis:
- the presence of alcohol within the cell makes a heavy demand of NAD+ and consequently the NAD+/NADH ratio falls. Other reactions that depend on NAD+ will thus be curtailed. In particular, the oxidation of lactate to pyruvate will become much slower than the reverse reaction, which accounts for the observed accumulation of lactate.
- Other redox reactions depending on NAD+ that occur in the tricarboxylic acid cycle become slower in the presence of alcohol. Therefore acetate is diverted into the fatty acid synthetase system accounting for the fatty liver condition commonly found in alcoholics.
- The hypoglycemia (a low blood glucose level) observed is the result of the failure of a metabolic pathway called gluconeogenesis
- Alcohol increases urine output by depressing the pituitary gland’s production of the antidiuretic hormone vasopressin. The resulting loss of body water takes with it important minerals such as potassium, magnesium, calcium and zinc.
- Alcohol is directly toxic to skeletal and cardiac muscles causing weakness and makes heart disease more likely, probably raising the blood pressure. The heart of an alchoholic appears bloated and weighs twice as much as a normal heart.
Effects of alcohol on pregnant women
Alcohol and hormonal production
maternal alcohol consumption during pregnancy can interfere with fetal development, not only directly through adverse effects exerted by alcohol that crosses the placenta and enters the fetal bloodstream, but also impairing
i) the functioning of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress;
ii) the hypothalamic-pituitary-gonadal axis, which controls reproductive functions;
iii) and the hypothalamic-pituitary-thyroid axis, which regulates the metabolism of almost all tissues.
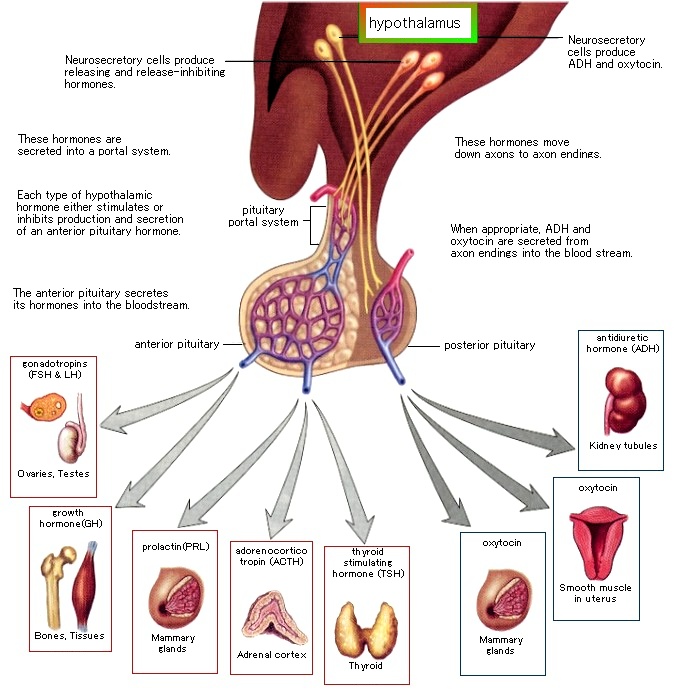
Alcohol consumption activates the HPA axis and stimulates glucocorticoid release, which can cross the placenta, resulting in elevated glucocorticoid levels in the fetal blood, signaling the fetal HPA axis to decrease its activity. At the same time, however, alcohol itself also crosses the placenta and directly activates the fetal HPA axis. Such conflicting messages may disrupt communication among the CNS, hypothalamus, pituitary and adrenal glands. (The hormonal effects of alcohol use on the mother and fetus, 1998)
Alcohol direct neurotoxicity and growth impairment
Ethanol inhibits insulin-mediated actions in the developing brain by reducing insulin and insulin-like growth factor (IGF-I) receptor tyrosine kinase (RTK) activities, leading to inhibition of glucose transport and ATP production and steady-state levels.
Studies correlated these adverse effects of ethanol with inhibition of growth-factor-stimulated survival signaling. In the developing CNS, insulin and insulin-like growth factor type 1 (IGF-1) receptors are abundantly expressed, and critical mediators of neuronal growth, viability, energy metabolism, and synapse formation. These processes are accomplished through complex pathways, beginning with ligand binding and activation of intrinsic receptor tyrosine kinases (RTKs), which phosphorylate the insulin receptor substrate types 1 (IRS-1) and 2 (IRS-2), and ending with activation of the p85 subunit of phosphatidylinositol-3 kinase (PI3 kinase) [25]. p85 eventually stimulates glucose transport [26] and inhibits apoptosis by activating Akt/protein kinase B (and therefore inhibiting the pro-apoptotic protein BAD) or inhibiting glycogen synthase kinase-3b (GSK-3b)

Neuronal loss associated with microencephaly in ethanol-exposed fetuses may therefore be caused by ethanol inhibition of insulin/IGF- 1-stimulated survival mechanisms, mediated by apoptosis or mitochondrial dysfunction. In fact, high levels of GSK-3b activity or activated BAD are associated with increased neuronal death.
(Ethanol inhibits insulin expression and actions in the developing brain, 2005)
Role of folate, choline and vitamin A

There is a growing body of evidence to suggest that choline; folate and vitamin A pathways in the central nervous system are greatly perturbed with the introduction of alcohol. Namely, the effects of alcohol are not uniform since some areas of the same region are more vulnerable to the effects of alcohol than others.
Further research has shown that animals subjected to ethanol in utero when supplemented with high levels of choline, folate or vitamin A show a reduction in the behavioural and physical characteristics of FAS. Although there are no studies examining the benefits of supplementing a combination of these three nutrients, supplementation of folate, choline and vitamin A to mothers may lessen the effects of alcohol in utero and aid in abolishing the most common neurodevelopmental disease-FAS.
Folic acid deficiency and FAS
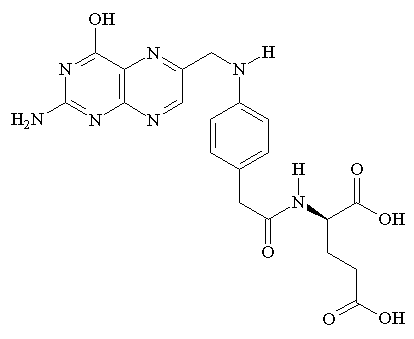
During pregnancy, the demand for folic acid increases since the fetus requires this nutrient for
DNA synthesis and cell proliferation. The placenta concentrates folic acid into the fetal circulation 2 to 4 times more than maternal blood concentration. Women who have folic acid deficiency during pregnancy, are more likely to give birth to premature and low weight infants with neural tube defects.
Folic acid, which is converted to tetrahydrofolate (folate), is primarily involved in moving single methyl groups around the cell . This ability has implicated folate as a key coenzyme in multiple metabolic pathways and in the methylation of DNA, which results in variations in protein expression. Upon the introduction of ethanol, the intestinal absorption, cellular and placentar entry of folate and folate metabolism are all greatly inhibited. (Ethanol and fetal nutrition: effect of chronic ethanol exposure on rat placental growth and membrane-associated folic acid receptor binding activity, 1985)

Ethanol directly inhibits the level of RNA transcription of multiple enzymes, for example methionine synthase (MS), S-adenosyl homocysteine (SAH) hydrolase, methionine adenosyltransferase III and methylene tetrahydrofolate reducatase (MTHFR), important in the production of methyl donors. In terms of DNA synthesis, the lack of folate leads to a decrease in DNA precursors, which subsequently leads to a decrease in cell division and growth restriction of both intrauterine and extra uterine growth.
The undermethylation of great spans of DNA that would normally be methylated is then transcribed and translated, producing proteins that would not normally be expressed.
Altogether there appears to be a clear but yet unknown role of epigenetics in FAS.
Folic acid is also important as an antioxidant during pregnancy.
Alcohol consumption during pregnancy creates oxidative stress to both the placenta and fetus. Formic acid, the toxic metabolite of methanol, has been reported in the umbilical cord blood from pregnancies with heavy amounts of alcohol consumption, and it is known to lead to neurotoxicity and oxidative stress. Folic acid is required for the detoxification of formic acid. Proper placental transfer of folic acid is critical to proper fetal development and can influence the fetal effects of alcohol. (Protective effect of folic acid against oxidative stress, 2001)
Given that poverty is a common risk factor for folate deficiency and fetal alcohol syndrome, the connection between folic acid and ethanol induced epigenetic changes may be worth exploring.
(Vitamin A, folate, and choline as a possible preventive intervention to fetal alcohol syndrome, 2012)
Choline deficiency and FAS
Choline and its metabolites are important for three main cellular processes: neurotransmission, namely through acetylcholine; structural integrity of cell plasma membranes and cell signalling; and in folate independent pathway as a methyl donor via its metabolite-betaine.
It is produced via de novo biosynthesis through the methylation of phosphatidylethanolamine (PE) to phosphatidylcholine (PC). However, de novo synthesis of choline alone is not sufficient to meet human requirements.

- Choline demonstrates its largest intracellular importance in cellular membrane composition and signalling. It is converted to phosphatidylcholine (PC), which is incorporated into the cell membrane and converted subsequently to phospholipase D (PLD) for cellular signalling. Ethanol competes with water for the PLD catalyzed reaction of PC and causes significant perturbations in the PLD signalling pathways.
- Choline is also a precursor to acetylcholine and in the developing brain it is used as a growth factor and has been implicated in both gastrulation and neurulation. This suggests that acetylcholine has significant implications in plasticity and adaptability in the postnatal animal. It is known that ethanol directly inhibits G-coupled muscarinic receptors and impairs acetylcholine synthesis through decreased available choline.
Interestingly one study in 2009 found that rats exposed to ethanol in utero and were supplemented with choline almost completely mitigated the effects of the ethanol on development and behaviour.
Vitamin A deficiency and FAS
Vitamin A deficiency is common worldwide. Vitamin A, also known as all-transretinal, is involved in two major processes: the vision cycle and cellular signalling. Retinal, by a two-step reaction involving retinal dehydrogenase (an enzyme that experiences ethanol inhibition), can also be converted to retinoic acid, which is biologically active and implicated in the up regulation and expression of hundreds, if not thousands, of genes.

Vitamin A metabolism
Vitamin A has three active forms (retinal, retinol and retinoic acid) and a storage form (retinyl ester): the parent compound retinol and its oxidation product, retinoic acid (RA), are shown in the stretched-out all-trans-configuration.

The first step in RA synthesis is the reversible oxidation of retinol to retinaldehyde. Most of the retinaldehyde in the embryo is generated by alcohol dehydrogenases that are competitively inhibited by ethanol. It is thought that the abnormalities evident in fetal alcohol syndrome result from the inhibition of RA synthesis by ethanol, because of the similarities between the embryonic abnormalities evident in RA teratogenicity and in fetal alcohol syndrome.
The embryonic enzymes that catalyze the irreversible oxidation of retinaldehyde to RA are predominantly of the cytosolic class I aldehyde dehydrogenase family (22, 177).

RA and neurogenesis
Homeobox genes have fundamental roles in the organization of developmental pattern in both invertebrate and vertebrate embryos and retinoids have been shown to affect the expression of Hox genes in vitro, in teratocarcinoma cells, and in early embryos (Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome, 1996) acting as an early developmental signal, possibly conditioning the posterior-to-anterior gradient in the gastrula(i.e. making sure that an animal forms both a head and a foot).
Retinoic acid affects neurons at various stages of differentiation, generating neurites and guiding them to various targets.
Alcohol's bad influence
Alcohol is thought to have a direct affect on retinoic acid metabolism through direct inhibition of alcohol or acetaldehyde dehydrogenase. This decreases the amount of retinoic acid available to produce the effects on neural development described above. (Assessing teratogenic changes in a zebrafish model of fetal alcohol exposure, 2012)
It is believed then that application of retinoic acid during gastrulation and somitogenesis may significantly decrease the degree of birth defects normally caused by ethanol.
"For the first time, our studies have demonstrated that high-dose ethanol affects the expression and activation of RA receptors, which could impair the signaling events and induce harmful effects on the survival and differentiation of cerebellar granule cells. Taken together, these findings could provide insight into the treatment options for brain defects caused by excessive ethanol exposure, such as in Fetal Alcohol Spectrum Disorders." (Ethanol impairs activation of retinoic acid receptors in cerebellar granule cells in a rodent model of fetal alcohol spectrum disorders, 2010)