Congenital heart block is a rare disorder, with an incidence of about 1 in 22000 live births. It is frequently associated with underlying structural congenital heart disease. When diagnosed in the postnatal period, approximately one-third of cases of congenital conduction system disease have associated structural disease. In utero, diagnosis of congenital heart block is associated with structural heart disease in approximately one half of the cases. There is a higher association of congenital heart block occurring with congestive heart failure in utero, and thus a poorer prognosis. In this case early diagnosis is very important, in order to use an aggressive therapy if necessary.
Electrocardiogram showing first degree atrioventricular block (PR= 160 msec, heartrate= 170 bpm) in a newborn.

Congenital Heart Block in Neonatal Lupus
Congenital complete heart block is the most common cardiac rhythm abnormality that presents itself with Neonatal Lupus (Neonatal Lupus, 2007); this disease is considered a form of passively acquired autoimmune disease in which maternal autoantibodies to the intracellular ribonucleoproteins Ro (SS-A) and La (SS-B), cross the placenta and injure the previously normal fetal heart. (A review of congenital heart block, 2003) These autoantibodies are members of the ANA family, anti-nucleus antibodies; particularly, anti-Ro/ SSA and anti-La/ SSB are typical of the Sjogren’s Syndrome.

A disease model of Sjögren's syndrome in a minor salivary gland. Virus infection leads to interferon- production by plasmacytoid dendritic cells and causes apoptosis in epithelial cells, exposing SSA/SSB and ribonucleoprotein in apoptotic blebs. B cells produce autoantibodies against these RNA-binding proteins and immune complexes are formed that bind to Fc gamma receptor IIa on plasmacytoid dendritic cells, which are stimulated to produce interferon-. Produced interferon- stimulates maturation of dendritic cells, activation of T cells and autoantibody production by B cells, creating an autoimmune vicious circle.
DC, dendritic cell; Fc RIIa, Fc gamma receptor IIa; IFN- , interferon- ; PDC, plasmacytoid dendritic cell; RNP, ribonucleoprotein.
A disease model of the Sjogren's Syndrome, 2006
Women with serum titers of anti-Ro antibody carry a 3% risk of having a child with neonatal lupus syndrome. If she had a prior experience with affected fetuses, her risk rises to about 18%.
DIAGNOSIS of CONGENITAL HEART BLOCK
Congenital heart block associated with Neonatal lupus is usually diagnosed in the presence of a slow heart rate discovered in a fetus or newborn in the absence of associated structural cardiac abnormalities. The majority of cases of congenital heart block, diagnosed in utero are detected by either auscultation or routine obstetrical ultrasound in low risk pregnancies. The diagnosis is confirmed by the performance of maternal fetal monitoring (MFM) and a fetal echocardiogram with Doppler techniques. In the past, only second or third degree block would be clinically manifest as a bradycardia.
The purpose of the fetal echocardiogram is to determine the level of block and also to rule out major associated structural lesions of the heart, such as left atrial isomerism with or without atrioventricular septal defects, and ventricular inversion, which are structural diseases associated with the presence of heart block without antibodies. The fetal echocardiogram is also able to detect any associated myocarditis by looking for the presence of decreased contractility on fetal echocardiogram as well as any secondary changes of cardiac enlargement, tricuspid regurgitation, pericardial effusion, or the development of hydrops fetalis.
A review of congenital heart block, 2003

Hydrops fetalis. Transverse section of fetal thorax displaying 4 chamber view of heart surrounded by pleural and pericardial effusions. Lungs are collapsed.
Maternal serum testing subsequently reveals antibodies to Ro and/or La, usually evaluated by ELISA testing. While the mother may have systemic lupus or other autoimmune diseases such as Sjogren's Syndrome, approximately half of the women at the time of diagnosis are asymptomatic.

In utero, the peak onset of the diagnosis of bradycardia is between 18 and 24 weeks of gestation, corresponding to the window of opportunity about six weeks after effective placental transport of maternal IgG antibodies begin.
PATHOPHISIOLOGY of CONGENITAL HEART BLOCK
The mechanism of causation of Congenital Heart Block is not completely understood but evidence points to the fetus beginning life with a normal cardiac structure and conduction system. At approximately 12 weeks of gestation (third month of pregnancy), maternal IgG antibodies against Ro and La intracellular ribonuclear proteins are actively transported across the placenta and are thought to bind specific cells of the fetal conduction system, causing, directly or indirectly, the heart damage; there are two different hypothesis about this:
- The first, hypothesis of the myocarditis, assumes that the antibodies cause an inflammation of the whole fetus’ heart, which may result in a cycle of inflammation, later scarring and fibrosis.
- The second one, electrophysiologic hypothesis, assumes that the antibodies cause an alteration of the electric state of cardiac cells.
The degree of heart block may vary from “first degree” to “third degree” block, but most cases diagnosed in utero present with a least second degree or more advanced block. There is a high mortality rate, particularly in fetuses diagnosed in utero with hydrops, and it is approximately 20%.
Hydrops fetalis (fetal hydrops) is a serious fetal condition defined as abnormal accumulation of fluid in 2 or more fetal compartments, including ascites, pleural effusion, pericardial effusion, and skin edema. In some patients, it may also be associated with polyhydramnios and placental edema. Hydrops fetalis, 2012
In those cases of autoimmune conduction system disease due to neonatal lupus, the bradycardia alone is not always the full extent of disease. Recently, there has been the recognition of a relatively high incidence of the development of late cardiomiopathy leading to heart failure, death or transplantation despite successful pacemaker implantation.
Late cardiomyopathy is associated with immune-related congenital heart block in 5-11% of cases. Other manifestations of Neonatal Lupus in the newborn involve other organs and systems:
- Cutaneous manifestations: appear within the second month of life of the newborn; are represented by the typical neonatal rash which appears as annular lesions, red and relieved, and especially in those regions exposed to light, like the face (tipically around the eyes), legs and arms. In the majority of cases they disappear spontaneously, without traces; at times some bright areas or dilated capillaries remain (teleangectasie).
- Blood alterations: appear at birth or during the first weeks of life; they are not severe and usually regress spontaneously. There may be a reduction of the amount of red blood cells (anemia), of white cells (leucopenia), of platelets (piastrinopenia).
- Hepatic alterations: appear at birth or during the first weeks of life and consist of an obstacle to the bile flux. Even though the lesion may be severe, it is reversible and children gain a normal hepatic functionality. There may even be alterations of the hepatic enzymes levels and jaundice.
While the non-cardiac manifestations of neonatal lupus are generally transient and resolve at approximately the time that the maternal antibodies are cleared from the infant's circulation at several months of age, the conduction system disease is essentially irreversible.

Skin rash, typical of the Neonatal Lupus
THERAPEUTIC APPROACH TO THE CONGENITAL HEART BLOCK DIAGNOSED IN UTERO
With increasing prenatal care and use of ultrasound technology in pregnancy, increasing numbers of cases of autoimmune congenital block are being diagnosed between 18 and 24 weeks of gestation. This raises the expectation for better prognostication and possibly for definite therapy. Based on the assumption that treatment for identified heart block in utero may be effective if it can reduce a generalized inflammatory insult and lower the titer of maternal autoantibodies, several prenatal therapeutic protocols have been utilized. These include the use of adrenocorticosteroids, which are not metabolized by the placenta, principally dexamethasone. Some researchers have also attempted plasmapheresis (separating plasma from blood cells by centrifugation) and the use of maternal alpha-adrenergic agents (elements which interact with the alpha-adrenergic receptor).
Therapeutic approach: if the heart block is already third degree and has been preent for three weeks or more, serial echocardiographic and obstetrical follow-up is provided, but no therapy is initiated. If, however, the third degree heart block has been recently diagnosed, the patient is offered a therapeutic course of dexamethasone 4 mg. orally once a day for a period of six weeks. If there are no changes in fetal status, the therapy is interrupted. On the other hand, if the fetus’ conduction system disease has improved to second degree block or better, the administration of dexamethasone continues until delivery and subsequently taper in the mother.
Dexamethasone is a synthetic member of the glucocorticoid class of steroid drugs, functionally corresponding to the endogenous hormones cortisol and cortisone, but with more specific pharmacokinetic and therapeutic properties and more moderate side effects. The clinical use of these molecules is due to their anti-inflammatory action, which occurs through the induction of the enzyme lipocortine; this enzyme can inhibit phospholipase A2 and the following cascade, which leads, through the action of lipoossigenasi and cicloossigenasi, to the production of inflammatory mediators. They also have an immunosuppressant action, probably due to the ability of dexamethasone to inhibit protein synthesis, leading to a reduced production of antibodies and to a reduction of the amount of lymphocytes, eosinophil granulocytes and basophil granulocytes. Dexamethasone is fastly absorbed by the gastrointestinal system so this kind of therapy is particularly effective, even though there are many side effects due to the complex mechanism of action.
Action mechanism of glucocorticoids, 2005
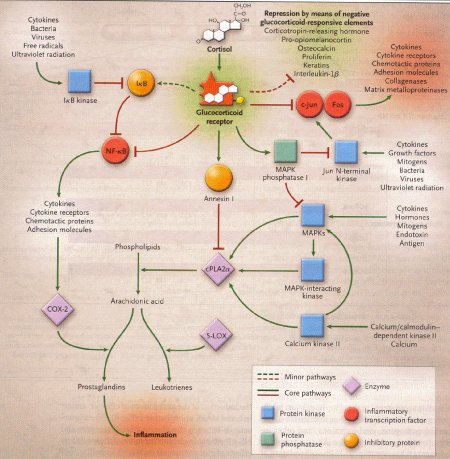
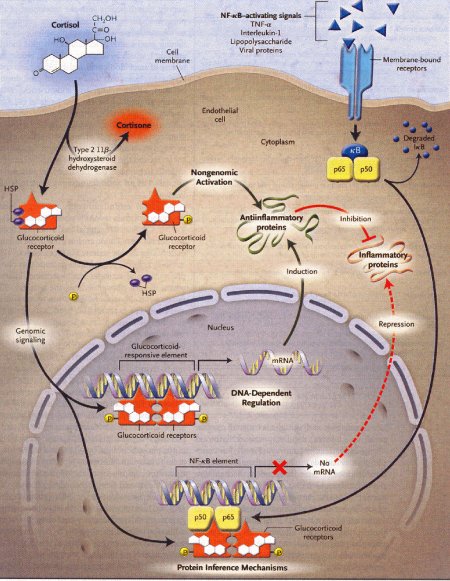
Occasionally, the fetal congenital heart block is associated with early signs of myocarditis and fetal hydrops; in these cases doctors use dexamethasone at 4 mg orally daily until improvement of the hydrops fetalis. Other therapies in such cases of hydrops have included plasmapheresis, maternal terbutaline, digoxin, diuretics or direct fetal pacing. There has been no long-term survival from these desperate measures, and therefore if the lungs are mature at this point, we would advise early delivery.
- TERBUTALINE: a bronchodilator drug which acts stimulating β2-adrenergic receptors located in the bronchi. (β2-adrenergic agonist).

- DIGOXIN: this molecule is a member of the class of digitalis glycosides. This drug is used to improve the power of contraction of myocardic fibers, both atrial and ventricular, with a inotropic positive effect. By inhibiting the Na+/K+-ATPase, cardiac glycosides cause intracellular sodium concentration to increase. This then leads to an accumulation of intracellular calcium via the Na+-Ca++ exchange system. In the heart, increased intracellular calcium causes more calcium to be released by the sarcoplasmic reticulum, thereby making more calcium available to bind to troponin-C, which increases contractility (inotropy). Inhibition of the Na+/K+-ATPase in vascular smooth muscle causes depolarization, which causes smooth muscle contraction and vasoconstriction. Mechanism of action of cardiac glycosides, 2012

The administration o dexametasone to the mother is not lacking of side effects; these include the risks associated with glucocorticoids:
- Increased infections
- Loss of bone density
- Diabetes
- Hypertension
- Cataracts
The fetal risks of maternal steroids include:
- Oligohydramnios (pathologic condition, typical of pregnancy, characterized by the reduction of the amniotic fluid which becomes less than 500 ml)
- Intrauterine growth retardation
- Adrenal suppression
There is also some suggestion of a risk to the developing fetal brain when exposed to steroids.
It is clearly advantageous to provide close fetal follow-up for monitoring the patient at risk for congenital heart block in the presence of maternal autoantibodies. Women more at risk are those with very high titers of anti-Ro and anti-La antibodies, as well as those with previously affected pregnancies. A new technique in fetal echocardiography has been recently developed: it allows to detect the first possible changes of fetal conduction system disease, that is, the presence of first degree heart block in the fetus. In this case, the overall fetal heart rate will still be normal, but this new non-invasive Doppler technique can measure the “mechanical PR interval” in the absence of an electrocardiogram. This will allow the earlier diagnosis and the possibility of very early treatment, which may be able to reverse the disease. For this reason, weekly fetal echocardiograms with Doppler are strongly recommended for pregnancies at risk.
The therapeutic approach to the newborn after birth has some more options, for example pacemakers for babies with significant bradycardia, such as those with a heart rate less than 55 bpm can be offered. Among all the patients in which a congenital heart block has been detected, more than 60% were provided with a pacemaker before reaching adulthood.

Neonates at risk for developing lupus rashes should be protected from sun exposure, but otherwise treatment is fairly conservative with the use of topical corticosteroids. The liver enzyme abnormalities and blood count irregularities are usually self-limited and require no specific treatment.
A review of congenital heart block, 2003