Abstract
Despite there are a lot of ongoing trials studying drugs for the Idiopathic Pulmunary Fibrosis (IPF) still there is not a cure. By the moment that NAC-based therapy is commonly used to treat IPF patients and the results of a 2012 study went in this direction, and finally the PANTHER-IPF study results, regarding the NAC monotherapy arm, are about to be published, we decided to collect and analyze all the data that we could find on PubMed and make a sum of them all. At the end of this work we can strongly recommend further investigation on the NAC monotherapy in IPF patients since different studies give us good results on this direction.
Background
Idiopathic pulmonary fibrosis is a progressive and fatal lung disease for which, nowadays, there is not an efficient treatment: several retrospective longitudinal studies suggest a median survival time from 2 to 3 years from the time of diagnosis depending on the histopathologic patterns.
The aim of this work is to collect studies above the efficacy of N-acetylcysteine (NAC) as a valiant treatment of IPF.
Results
Nowadays there are not enough statistically significant studies on the NAC monotherapy conducted in human, also because of the lack of patients . Statistically significant there is just the 2012 Japan RCT that showed that NAC therapy was associated with stability of FVC, the PANTHER one is ongoing . Furthermore studies conducted in vivo and in vitro support the theory that this treatment has to be studied also for the lack of side effects that are the reason of the Pirfenidone therapy suspension.
Introduction
Idiopathic pulmonary fibrosis (IPF) is defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and limited to the lungs. It is characterized by progressive worsening of dyspnea and lung function and is associated with a poor prognosis and an estimated median survival of only 3 years after diagnosis.
Pirfenidone is an inhibitor of fibrosis and collagen production induced by TGF-beta and, at the same time, it reduces the production of TNF-alfa and IL-1beta implicated in the inflammatory process, actually is the only worldwide drug approved for the IPF patients treatment.
The Pirfenidone is discontinued by patients because they suffer side effects, mostly gastrointestinal. The median survival after the start of Pirfenidone was 3.8 years (only 8 months more than the median overall survival). These are the reasons why some studies about another kind of treatment with N-acetylcysteine have been done and others are now ongoing, so we decided to make a little review.
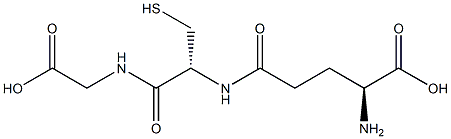
N-acetylcysteine (NAC) also known as N-acetyl-L-cysteine (abbreviated NAC), is a drug used primarily as a mucolytic agent, it is a thiol containing compound which by providing sulfhydryl groups, can act both as a precursor or reduced glutathione (GSH), in fact, acts as a nucleophilic scavenger and as an enzyme-catalyzed antioxidant in the event of electrophilic/oxidative tissue injury so it can interfere with several signaling pathways that play a role in the regulation of apoptosis, angiogenesis, cell growth, nuclear transcription and cytokine production . In humans NAC had been proven that improves idiopathic pulmonary fibrosis (IPF), various forms of alveolitis and it’s used to avoid hepatoxic effects of Paracetamol overdose since GSH depletion has been recognized as a hazardous condition and conversely, GSH rescue, meaning recovery of the protective potential of GSH by early administration of N-acetylcysteine (NAC), has been found to be life-saving. Therefore, GSH has a major role as a protector of biological structures and functions.
Overall, the anti-inflammatory action of NAC is well documented in vitro as well as in vivo.

N-Acetylcystein
Glutathione
Materials and methods
We decided to use the PubMed library and search: IPF[All Fields] AND NAC[All Fields] (18 results); IPF[All Fields] AND nac[All Fields] AND ("review"[Publication Type] OR "review literature as topic"[MeSH Terms] OR "review"[All Fields]) (4 results included in the first one)
Other researches were discarded because misleading like: IPF[All Fields] (2515 results), IPF[All Fields] AND ("therapy"[Subheading] OR "therapy"[All Fields] OR "treatment"[All Fields] OR "therapeutics"[MeSH Terms] OR "therapeutics"[All Fields])(1089 results).
All datas are referred to a research made the 6th June 2013
The studies
Since 1990 it’s known that GSH deficiency in respiratory-tract was observed in IPF and that was suppoused to create an environment favoring an excessive fibroblast proliferation in bronchoalveolar lavage fluid (BAL) or calf serum (CS) cells in vitro.
In 1994 the effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis were studied because of a deficiency of glutathione in the lung epithelial lining fluid (ELF) was observed. Following therapy with oral NAC, glutathione levels in bronchoalveolar lavage fluid (BALF) were significantly increased in comparison to pretherapy, whereas the increase in ELF levels was not significant. There were no relevant side effects caused by the therapy, and no variations were registered in routine clinical and bronchoscopic parameters. They concluded that is possible and safe to augment lung glutathione levels in IPF patients; thereby, potentially augmenting pulmonary antioxidant protection.
Another study conducted on 14 patients (8 with IPF and 6 controls) again by Meyer et al. demonstrated that 1.8g of NAC administered intravenously increases GSH both in ELF and BALF with no side effects.
Another study was conducted on human fetal lung fibroblasts (HFL-1) to assess the effect of N-acetyl-l-cysteine on excessive production of transforming growth factor-beta (TGF-beta) an important mediator of tissue remodeling or fibrotic processes observed in idiopathic pulmonary fibrosis (IPF). TGF-beta mediated tissue remodeling in fibroblasts and its modulation of fibronectin and vascular endothelial growth factor (VEGF), which are considered important mediators of tissue repair and remodeling, were also evaluated. In conclusion it was proved that NAC can affect the TGF-beta-induced tissue remodeling or fibrotic process in vitro abolishing the gel contraction and also fibronectin and VEGF production.
TGF-beta1 also induces alveolar epithelial-mesenchymal transition (EMT) in idiopathic pulmonary fibrosis patients as it resulted in an investigation conducted in a rat epithelial cell line (RLE-6TN) and in primary rat alveolar epithelial cells (AEC), in fact both kind of cells exposed to TGF-beta1 for 5 days underwent EMT and acquired a fibroblast-like morphology. These changes were inhibited by NAC so they concluded that NAC prevents EMT in AEC in vitro. That’s the reason why beneficial effects of NAC in IPF may be mediated by its effects on alveolar EMT.
The demonstration of NAC dose-dependent inhibitory effect on players of the etiopathogenesis of Interstitial lung disease, was conducted by considering the production of interleukin-8 (IL-8) and matrix metalloproteinase-9 (MMP-9) as well as intercellular cell adhesion molecule-1 (ICAM-1) expression. The study was conducted on bronchoalveolar lavage (BAL) cells of patients with IPF or sarcoidosis.
In 2009 it has been shown also that N-acetylcysteine inhibits TNF-alpha, sTNFR, and TGF-beta1 release by alveolar macrophages taken from the BAL of idiopathic pulmonary fibrosis patients cultured in vitro.
Li et al. induced IPF in rats with bleomycin and demonstrated that N-acetylcysteine alleviated this condition downregulating the lysyl oxidase activity.
NAC suppresses the action of NOX4 and so the TGF beta1 myofibroblast differentiation and PDGF-induced fibroblasts migration in IPF.
The expression of p63 in lung tissues affected by IPF and its key role in the vitro metaplasia model and, subsequently, its total suppression by N-acetyl-l-cysteine has been demonstrated by Murata et al., meanwhile dexamethasone or pirfenidone could not contrast the upregulation of this molecule.
In 2012 The Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis had been studied by Homma et al. in Japan with a multicentre, prospective, randomized, controlled clinical trial conducted to assess the efficacy of inhaled N-acetylcysteine (NAC) monotherapy in Japanese patients with early stage IPF.
Seventysix patients were randomly assigned to a NAC treatment group or to a control group that received no therapy. There were no significant differences in the FVC change between the two groups. Post hoc exploratory analyses showed that NAC therapy was associated with stability of FVC in patients with initial FVC <95% and in patients with initial diffusing capacity of carbon monoxide <55.
Conclusions
Despite the “weak no” recommendation given after the results given by the IFIGENIA study that investigated on a triple combination therapy , these findings indicate that NAC monotherapy may have some beneficial effects in patients with early stage IPF. Further trials on the IPF patients have to be done to demonstrate that inhaled NAC can be efficacious, also because, nowadays, NAC-based therapy is not approved, but it’s commonly used to treat this kind of patients. Discussion of these data and recent findings highlight the importance of a further update based on new studies.