What is NGF, structure and metabolic pathways
Nerve growth factor (NGF) is a small secreted protein that is important for the growth, maintenance, and survival of certain target neurons (nerve cells). It also functions as a signaling molecule. It is perhaps the prototypical growth factor, in that it is one of the first to be described. While "nerve growth factor" refers to a single factor, "nerve growth factors" refers to a family of factors also known as neurotrophins. Other members of the neurotrophin family that are well recognized include Brain-Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3), and Neurotrophin 4/5 (NT-4/5).
The NGF gene is located on the short (p) arm of chromosome 1 at position 13.1. ( http://www.ncbi.nlm.nih.gov/gene/4803 )

The structure of NGF was first solved by X-ray crystallography and published in 1991 by McDonald et al. in Nature. NGF forms a cystine knot structure made up of beta strands twisted around each other and linked by disulfide bonds. Most structures are dimeric. At the time this structure was solved, this fold had never been seen before.
NGF binds with at least two classes of receptors: the p75 LNGFR (for "low-affinity nerve growth factor receptor") neurotrophin receptor (p75(NTR)) and TrkA, a transmembrane tyrosine kinase. Both are associated with neurodegenerative disorders. ( http://www.jneurosci.org/content/18/9/3273.full.pdf )
NGF binds to high-affinity tyrosine kinase receptor TrkA. TrkA dimerizes and autophosphorylates its tyrosine kinase segment, which leads to the activation of PI 3-kinase, ras, and PLC signaling pathways. Alternatively, the p75NTR receptor can form a heterodimer with TrkA which has higher affinity and specificity for NGF.
Binding of NGF to the TrkA receptor causes activation of the receptor tyrosine kinase and downstream signaling cascades. One of the downstream signaling pathways of NGF activates phospholipase C, releasing DAG and IP3 and activating associated downstream pathways such as protein kinase C. Subsequently, phosphatidylinositol-3 kinase (PI3K) is activated, resulting in Akt kinase activation. Study results have shown that blocking PI3K or Akt activity results in death of sympathetic neurons in culture, regardless of NGF presence.However if either kinase is constitutionally active, neurons survive even without NGF.
Another NGF-activated pathway is the ras-mediated activation of the map kinase pathway. This pathway is initiated through recruitment and activation of Shc, which leads to ras activation through Grb-2 and Sos-1. The Map kinase cascade includes raf, Mek and Erk. Raf, in turn activates the MAPK cascade to facilitate ribosomal s6 kinase(RSK) activation and transcriptional regulation.
Both Akt and RSK, components of the PI3K-Akt and MAPK pathways respectively, act to phosphorylate the cyclic AMP response element binding protein (CREB) transcription factor.
The CREB family of transcription factors are involved in NGF-induced survival of sympathetic neurons. Further understanding of NGF signaling may be applied to the modulation of NGF responses in neurodegenerative conditions.

Clinical significance
ON THE BRAIN
NGF prevents or reduces neuronal degeneration in animal models of neurodegenerative diseases and these encouraging results in animals have led to several clinical trials in humans.NGF has also been shown to promote peripheral nerve regeneration in rats. The expression of NGF is increased in inflammatory diseases where it suppresses inflammation. Also, NGF appears to promote myelin repair. Hence NGF may be useful for the treatment of multiple sclerosis. NGF could also be involved in various psychiatric disorders, such as dementia, depression, schizophrenia, autism, Rett syndrome, anorexia nervosa, and bulimia nervosa. Dysregulation of NGF signaling has also been linked to Alzheimer's disease.
An increase in cortical and subcortical NGF has been found in patients with Alzheimer’s disease. Alzheimer’s is a neurodegenerative disease with which dysregulation of NGF signaling has also been linked to, causing impaired retrograde transport of NGF to certain areas of the brain. This impairment may be caused by an atypical production or use of receptors in the brain. A new treatment for Alzheimer’s includes stimulating NGF receptors via NGF infusion has been shown to increase blood flow and verbal episodic memory. These improvements have been longer lasting than other treatments for Alzheimer’s. ( http://www.adcs.org/studies/ngf.aspx)
Stress and/or anxiety are usually a precipitating factor in these disorders and affects levels of NGF, leading to impaired cognitive functioning.
Rett syndrome and Autism often show similar signs early in life, such as slowing development and intellectual disability. One distinguishing factor is that low levels of NGF have been found in the cerebral spinal fluid of those with Rett Syndrome compared to children with Autism who have relatively normal to high levels. Pharmaceutical therapies with NGF-like activity can be effective in treating Rett syndrome, including better motor and cortical functioning as well as increased social communication. (http://www.ncbi.nlm.nih.gov/pubmed/10210246 )
Impairment of neuroplasticity and altered levels of neuro-trophins are involved in Bipolar Disorder. NGF has been found to be decreased overall in Bipolar Disorder patients. This decreased NGF may serve as a biological marker when assessing the present state of a Bipolar Disorder patient.When Bipolar Disorder patients were treated with lithium, their NGF concentrations increased in the frontal cortex, limbic forebrain, hippocampus, and amygdala. (http://www.ncbi.nlm.nih.gov/pubmed/20923384 )
IN IMMUNE SYSTEM
NGF has also been suggested to play a role in other physiological systems and tissues such as the immune system. ( http://www.ncbi.nlm.nih.gov/pubmed/15349043: http://www.ncbi.nlm.nih.gov/pubmed/15349043 )
Cells of the immune-hematopoietic system produce and utilize NGF . Since the early description of the effects of NGF on mast cells, the role played by the neurotrophin in the regulation of immune functions and immune cells' behavior has been greatly characterized. NGF receptors are expressed in immune organs and on immune cell populations allowing NGF to modulate cell differentiation and regulate the immune response. NGF affects the survival and/or differentiation and/or phenotypic features of hematopoietic stem cells , granulocytes, lymphocytes and monocytes. NGF concentrations in the tissues change during inflammation and inflammatory mediators induce NGF synthesis in a variety of cell types . Enhanced production of NGF has been reported in inflamed tissues of patients with inflammatory and autoimmune diseases, but the reasons why NGF concentration is enhanced and how this can affect inflammatory responses are far from being fully understood.
In animal experiments, NGF induces degranulation of peritoneal mast cells and shape change of thrombocyte. Mast cells and NGF appear to be involved in neuroimmune interactions and tissue inflammation, with NGF acting as a general ‘alert’ molecule capable of recruiting and priming tissue defence processes following insult as well as systemic defensive mechanisms. Moreover, mast cells themselves produce NGF, suggesting that alterations in normal mast cell behaviours can provoke maladaptive neuroimmune tissue responses whose consequences could have profound implications in inflammatory disease states. ( http://www.sciencedirect.com/science/article/pii/S0166223696100588 )

A large body of evidence supports the notion that NGF’s primary function in adults is to mediate the inflammatory and immune response after tissue injury, especially to initiate and maintain hypersensitivity, a hallmark symptom of inflammation. This hypersensitivity manifests as increased sensitivity to a noxious stimulus (hyperalgesia) and/or the perception of a non-noxious stimuli as noxious (allodynia). One mechanism to account for this hypersensitivity is a reduced threshold for firing and an increase in the excitability of small diameter sensory neurons that communicate noxious information to the central nervous system. This phenomenon, termed peripheral sensitization, increases the firing of small diameter sensory neurons and results in an increase in transmitter release from peripheral and central terminals of these neurons (Figure 1⇓). The increased release of the peptide transmitters, substance P (SP) and calcitonin-gene related peptide (CGRP) from peripheral endings of sensory neurons contributes to neurogenic inflammation. ( I searched http://www.ncbi.nlm.nih.gov/pubmed/7704300 )
The ability of NGF to augment nociceptive responses has received much attention and has raised the idea that attenuating the actions of this neurotrophin might prove to be a novel therapy for the treatment of chronic pain. ( www.ncbi.nlm.nih.gov/pubmed/23157347 )
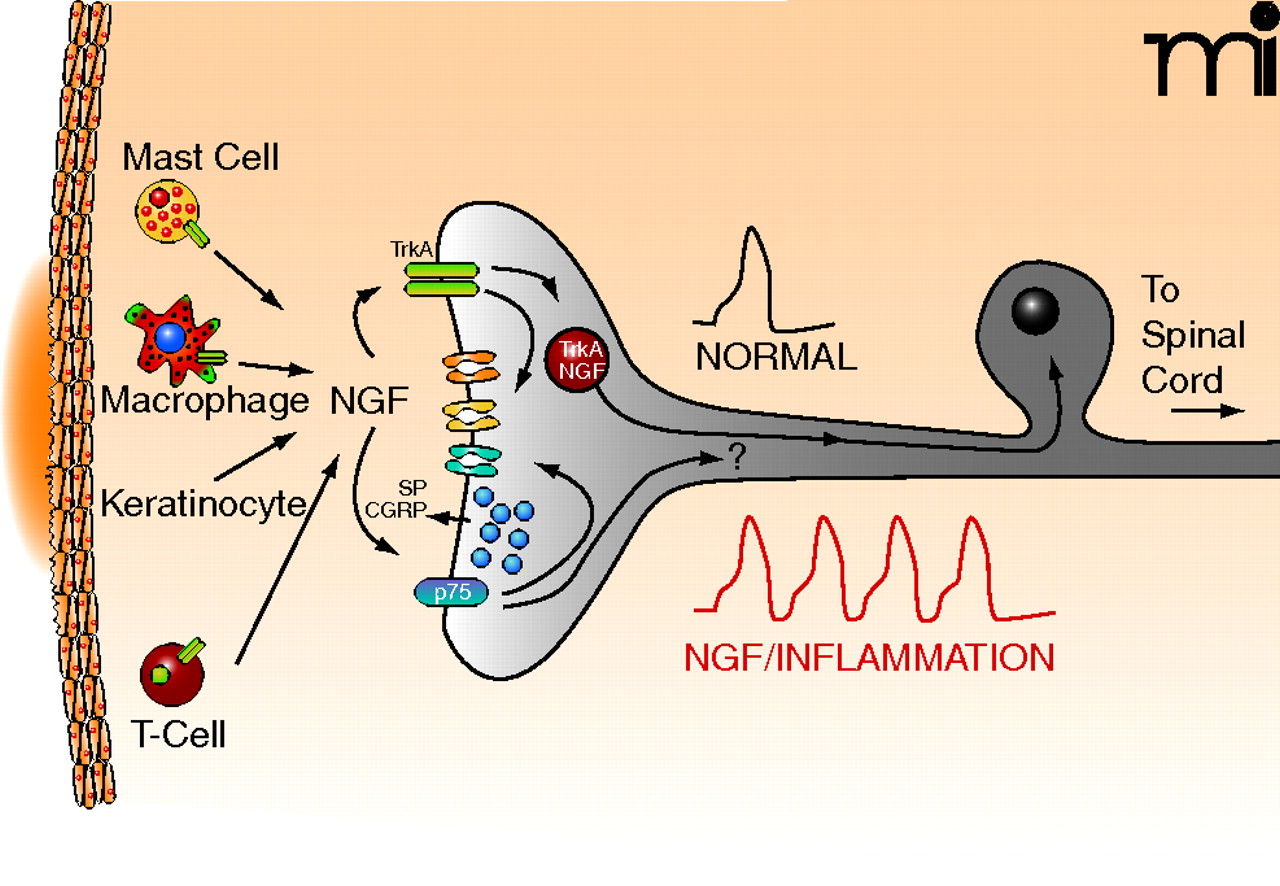
Release of NGF produces sensitization of the peripheral terminals of sensory neurons. Upon localized tissue trauma, mast cells, macrophages, keratinocytes, and T cells all release NGF, which can then interact with its receptors, TrkA and p75, on the nerve endings. Bindings to the NTR activates intracellular signaling cascades that can modulate the activity of different ion channels in the nerve ending or could enhance the release of the neuropeptides, substance P (SP) and/or calcitonin gene-related peptide (CGRP). Under normal conditions, a noxious stimuli might elicit a single action potential whereas after treatment with NGF or as a consequence of inflammation, the nerve now give rise to multiple action potentials. Upon binding the neurotrophin, the TrkA-NGF complex may be retrogradely transported back to the cell body where it can modify the expression of multiple genes, ion channels. It is possible that p75 lacking NGF can be transported back to the cell body where it also serves to alter gene expression. ( http://www.ncbi.nlm.nih.gov/pubmed/10393882 ) ( http://triggered.edina.clockss.org/ServeContent?rft_id=info:doi/10.1124/mi.7.1.6 )
Rita Levi Montalcini wrote: "Predictions of the unpredictable are encouraged by the same history of NGF which may be defined as a long sequence of unanticipated events which each time resulted in a new turn in the NGF unchartered route, and opened new vistas on an ever-changing panorama. This trend, which became manifest from the very beginning and in fact alerted me to the existence of NGF, is perhaps the most attractive, even though elusive trait of this thirty-live year long adventure. One can at present only predict where future developments are most likely to occur. The main causes of unpredictability of the findings, reside in the intricacy of the new surroundings where NGF is moving - the CNS and the immune system-rather than in NGF itself. The enormous complexity of these two networks, which on the basis of recent findings are closely interrelated and influence each other through bidirectional signals, opens endless possibilities for NGF activation of distinct repertoires of cells belonging to one or the other system. How many indirect effects can be elicited by direct NGF action on cholinergic, adrenergic and peptidergic neurons interlinked via fiber pathways and humoral channels or through short-distance diffusion?
Likewise, how many effects could follow the simple histamine release by NGF activated mast cells, considering the well-established role of this amine as animmunomodulator or an immunosuppressor?
These considerations hold also for the potential utilization of NGF in brain and immunosystem disorders. For instance, whenever cell death of specific neuronal populations may be linked to a decreased local availability of neurotrophic factors, such as NGF, its exogeno-us supply or stimulation of its endogenous production via pharmacological agents may offer a promising approach to presently incurable diseases."
(http://www.nobelprize.org/nobel_prizes/medicine/laureates/1986/levi-montalcini-lecture.pdf )
HYPOTHALAMIC INVOLVEMENT IN THE ACTIVATION OF THE PITUATARY -ADRENOCORTICAL AXIS BY NERVE GROWTH FACTOR
Is love just a chemical?
Intravenous injection of nerve growth factor (NGF) into rats produces a dose-dependent (from 0.1 to 5 nmol/kg) increase in circulating concentrations of adrenocorticotropin (ACTH) and corticosterone. We have investigated whether this effect is produced through a direct action on a component of the hypothalamo-pituitary-adrenocortical axis. ( http://www.ncbi.nlm.nih.gov/pubmed/8264866 )
Although the current knowledge of the neurobiological substrates of love in humans remains meagre, studies of pair bonding in sheeps and voles havereported a recurrent association between high levels of activity in the hypothalamic pituitary adrenal (HPA) axis and the subsequent expression of social behaviours and attachments. Accordingly, central neuropeptides, and especially oxytocin and vasopressin, which have been heavily implicated in increasing positive social behaviours in animal studies may be also central players in the regulation of the HPA axis.
It seems therefore reasonable to hypothesize that neurochemical mediators capable of modulating HPA reactivity can also be involved in the formation of social bonds.
This is, to our knowledge, the first study investigating the peripheral levels of neurotrophins in subjects in love. The main finding of our study is that levels of NGF are significantly elevated in the early phase of a romantic love. Notably, we have also demonstrated that, at the beginning of a romance, subjects in love show a significant positive correlation between levels of NGF and the intensity of romantic feelings. Although the mechanisms behind this selective increase of NGF remain to be determined, our data suggest thatraised NGF levels when falling in love could be related to specific emotions typically associated with intense early-stage romantic love, such as emotional dependency and/or euphoria. ( http://www.byz.org/~david/neuro/NGF%20and%20romantic%20love.pdf )
Is this the secret of eternal life?
Most centenarians attribute their great age to some magic elixir or other. The longevity of the Italian scientist Rita Levi-Montalcini, who this week became the first Nobel Prize-winner to reach the age of 100, might be the result of a potion that is a little out of the ordinary: Professor Levi-Montalcini, it is said, puts her undiminished mental vigour down to regular doses of nerve growth factor (NGF).
According to Pietro Calissano, who collaborated with the professor on an article for Scientific American in which she announced her discovery in 1979, NGF may have played a direct role in her amazing vitality. "Every day, she takes NGF in the form of eye drops," he said, "but I can't say for sure if this is her secret. At the start, it seemed this molecule's effect was restricted to acting on the peripheral nervous system, but then it emerged that it has a very important role in the brain. Contrary to what was believed, the brain does not have a rigid structure but is in continuous movement, and NGF helps neurons – which we begin to lose between 10 and 15 years old – survive."