DEFINITION
Primary pulmonary hypertension (PPH) results from occlusion of lung arteries or veins or capillars which increases the resistance to blood flow through the lungs.
There is an anormal endotelial proliferation and resulting intimal layer hypertrophy.
EPIDEMIOLOGY
This is a rare condition that affect 2 patients to 1 milion, predominatly female sex over 40 years old.
PATHOGENESIS
This is a condition that increase average blood lung pressure over 25 mmHg misured by Swan-Ganz Catheter. and can lead hearth failure.
Primary Pulmonary Hypertension belongs to group I of WHO classification
This is ARTERIAL Hypertension and involves in vasocostriction. There are two main types:
In the first one, causes are no known but recent studies have dimostrated a correlation with potassium gene mutations KCNK3
In the second one, there is a mutation of BMPR2(autosomic dominant): This is Bone
morphogenetic protein receptor, member of TGF beta superfamily.
SYMPTOMS
The symptoms are nonspecific and include breathlessness, fatigue, weakness, angina, syncope and abdominal distension .
The physical signs of Pulmonary Arterial Hypertension include left parasternal lift, an accentuated pulmonary component of second heart sound, a pansystolic murmur of tricuspid regurgitation, a diastolic murmur of pulmonary insufficiency and an RV third sound. Jugular vein distension, hepatomegaly, peripheral oedema, ascites and cool extremities characterise patients in a more advanced state. Lung sounds are usually normal. The examination may also provide clues as to the cause of PH. Telangiectasia, digital ulceration and sclerodactyly are seen in scleroderma, while inspiratory crackles may point towards interstitial lung disease .The stigmata of liver disease such as spider naevi, testicular atrophy and palmar erythema should be considered.
PATIENT RISK FACTORS
Pulmonary Hypertension is multifactorial desease
that can be related with:
•Autoimmune diseases that damage the lungs, such as scleroderma , systemic sclerosis. and rheumatoid arthritis
•Blood clots in the lung (pulmonary embolism)
•Congestive heart failure
•Heart valve disease
• HIV infection
•Low oxygen levels in the blood for a long time (chronic)
•Lung disease, such as COPD or pulmonary fibrosis
•Obstructive sleep apnea
Genetic factors
-IDIOPATIC PULMONARY HYPERTENSION
This is often correleted with genetic mutations such as KCNK3 gene potassium channel localized in locus 2p23. It belongs to Potassium subfamily member 3 ( on total of 15 ), 2 linked transmembrans 4 multisteps proteins.

There are three main potassium channel Family: 6STM, 4STM, 2STM.
Leak channels belongs to 4STM
KCNKs are called leak channels because they allow K+ to leak down its concentration gradient in
all citoplasmatic membrane cells. Their name is derived from the fact that the α subunits consist of
four transmembrane segments, each containing two pore loops. This structure originates selective filter. Negative Aminoacidis are localized in cytosolic side and permits to get near
cations.Potassium ions go out of cell through selectice filter and interacts with four carbonilic
oxigen moleculars.
KCNKs are mainly located in citoplasmatic membran of miocities in pulmonary artery.
In normal conditions, these channels stay always open because they have NOT inactivation gates.
KCNKs are responsable of leakage conductance that generates membrane potential at rest (resting potential) togheter with Na , Cl leak channel. They modulate timing a and potential action frequence.
In a typical cell at rest, membrane has selective potassium permeability and resting potential is about -60 mV , that is more positive than equal potential (-90 mV). In this normal condition, potassium goes out of cells at rest.
In patients whith KCNK mutations, Potassium channel have not a correct selectivity filter so potassium ion can't go out of cells. Consequently, resting potential is more than -60 mV. Calcium voltage dipendent channels are activated, so miocities are excited start contractioning. This situation lead to vasocostriction.
There are six KCNK mutations (heterozygous missense variants).The most important is heterozygous missense variant c.608 G→A (G203D),a missens mutation that determinetes a riduction of potassium current because leak channel have been inactivated. This is heterozygous c.608G-A transition in the KCNK3 gene, resulting in a gly 203-to- asp (G203D) substitution at a highly conserved residue in the second pore region of the protein, which is critical for the gating function of the potassium channel.
Potassium leak channel are essential for manteining Polmonary artery vascular tone , so its malfunction can lead to serious problems, such as right ventricle disfunction.
-FAMILIAR HYPERTENSION
This is correleted with BMPR2 , a member of serine/threonine receptor kinasis (type 2)
that belongs to TGFbeta superfamily.
BMPs are dimeric ligands involved in endotelial cell growth.
They are cytokines of BMPR through the formation of eterodimer receptor formed by type one and type two that activated TGFbeta pathway
Smad 1/5/8 (Smad family protein) are phosphorilated and activeted. Then there is a formation of
heteromeric complex with R-smad 4 (Co activator), then it traslocates in the nucleus and activates oncosopressor genes.
R-Smad/Smad4 Complex regulates a sequence called Smad Box. It is charaterized by 4 bases (5'-
GTCT-3') localized in TGF Beta promotor.
Patients with TGFbeta way mutations have an increase of cell proliferation, lead to ipertrophy of artery layers.
BMPR2 mutations and include termination, frameshift, and nonconservative missense changes in amino acid sequence Researchers have identified more than 140 BMPR2 gene mutations that cause pulmonary arterial hypertension.
Vascular factors
There are 3 main pathways involved in regulation of pulmonary vascolar tone:
Endothelin pathway pathway. Endothelin a causes vasocostriction in pulmonary artery.
Prostacyclin Pathway pathway.
Nitric oxide pathway oxide pathway.
Pulmonary Hypertension is also related to endothelial dysfunction: there is a stimulation of
vasocostrictors such as VEGF and thromboxane and a decrease of vasodilatator such as nitric oxide,
that cause vasoconstriction and smooth muscle and advential hypertrophy.
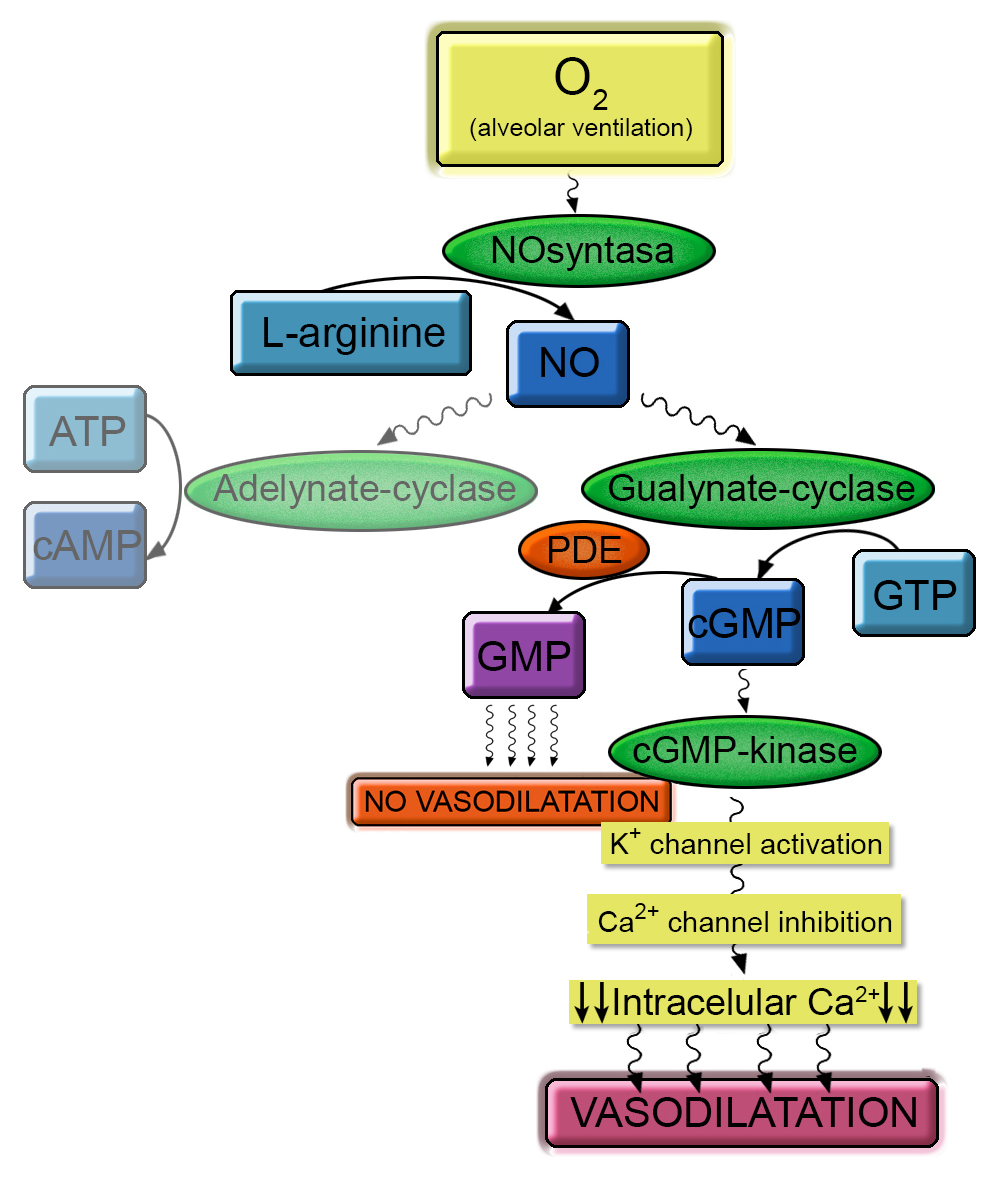
Normally, in the vascular endotelium, nitric oxide synthase produces nitric oxide from arginina.
Then NO activates guanylate cyclase that stimulates cGMP production from GTP.
cGMP activates serine threonine chinasis that activates potassium channel and subsequently
calcium channel. Finally there is a riduction of intracell calcium, essential condition for
vasodilatation.
PDE5 (Phosphodiesterase type V) is a metalohidrolase (Zn dipendent) that idrolase cGMP in GMP.
It is essential to reduce vasodilatation mediated by cGMP.
Patients with pulmorary ipertension have a riduction of NO syntetasy activty . Causes are not
known. Probably arginin deficiency could be a factor.
Furthermore, in pulmonary arteries, PDE5 is abundant; this is condition that favourite
vasocostriction.
Metabolic Factors
Homocysteine
(normal level 5-12 µmol/L) derived from mhetionine. It is an essential amminoacid.
High Homocysteine levels cause endothlial disfunction (Possible arterial thrombosis ) and hyperplasy of smooth cells
Infact Homocysteine inhibits the expression and activity of endothelial cell surface thrombomodulin
Possible causes:
-deficit of vit B12 or B6
-methylenetetrahydrofolate reductase gene C677T polymorphism
Acquired Factors
*Smoking
*After Splenectomy.
*Using Birth control pill that increase risk of thrombosis and embohlism
Hormonal Factors
17β-estradiol
(E2) causes mitogenic
angiogenic, and antiapoptotic effects in pulmonary artery.
COMPLICATIONS
After cronic increasing of pulmonary arterial pressure, Right heart has a riduction of his compliance so it has a disfuction during thelediastolic phase.Consequently, there is an increasing of right atrial pressure with Hypertrophy of miocardic layers.
Aterward, there is right ventricular insufficiency.
DIAGNOSIS
Diagnosis. consist of sequent steps:
- Clinical Symotoms
- Electrocardiogram:The ECG may provide suggestive or supportive evidence of PH by demonstrating RV hypertrophy and strain, and right atrial dilatation.
- Chest radiograph:In 90% of patients it is abnormal at the time of diagnosis. Findings include central pulmonary arterial dilatation, which contrasts with ‘pruning’ (loss) of the peripheral blood vessels. Right atrium and RV enlargement may be seen in more advanced cases.
- Pulmonary function tests and arterial blood gases.
- Transthoracic echocardiography.Two-dimensional echocardiography with Doppler flow studies is the most useful imaging modality in patients with suspected pulmonary hypertension.
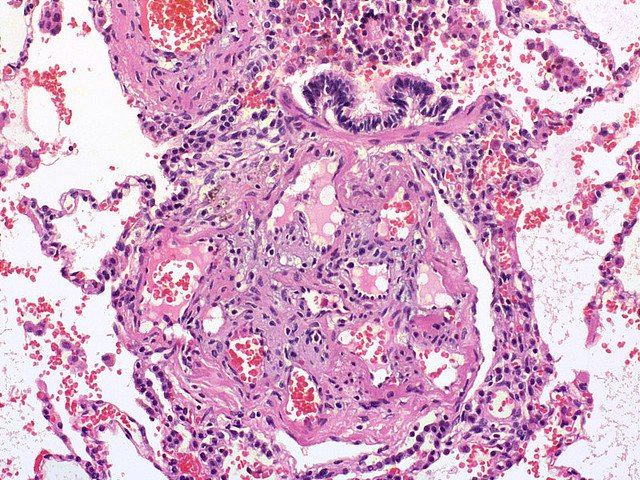
- histopathology:Intimal lesions consist of eccentric intima thickening, and fibrotic, plexiform, concentric, and dilation/angiomatoid lesions. There are Plexiform lesions, typically located in branching pointes of muscular arteries, consists of a network of vascular channels lined up by endothelial cells .
THERAPHY
In idiopatic pulmonary ipertension, cell colture studies have dimostrated that KNCK mutation effects can be stopped by using a fosfololipasy A2 inibitor called ONO-RS-082. It Inhibits
epinephrine-stimulated thromboxane production (86% at 3.5 μM) via inhibition of PLA2 in human platelets.
Inhibition of PLA2 by ONO-RS-082 regulates water channel aquaporin-1. Disrupts membrane tubule-mediated reassembly and maintenance of the Golgi complex.
The treatment consist of:
using vasoactive substance, that reduce smooth muscle contraction. Such as:
-prostaglandins (prostaglandin I2) such as Flolan.
that requires continuous infusion through central venous catheter.
-Phosphodiesterase type 5 inhibitors PDE5
such as sidenafil
-Endothelin receptor antagonists, that block the action of ET A receptor type A
that cause vascular smooth muscle contraction
-Activator of guanylate cyclase.
-Calcium antagonist
Surgical :
- septostomy
-Lung transplantation