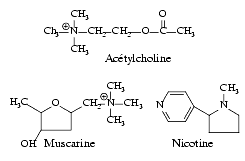
Acetylcholine (often abbreviated ACh) is a neurotransmitter in both the peripheral nervous system (PNS), including Parasympathetic System, and central nervous system (CNS) in many organisms including humans.

Acetylcholine has muscarinic and nicotinic effects by stimulation of the corresponding receptors.
Acetylcholine synthesis
Acetylcholine (Metabocard HMDB00895) synthesis requires
Recycling

Recycling pathway of acetylcholine (ACh) synthesis, release, action and breakdown at a cholinergic nerve terminal. AChE acetylcholinesterase, BChE butyrylcholinesterase, ChAT choline acetyltransferase, CHT1 high-affinity choline transporter-1, M muscarinic receptor, G-protein coupled, N nicotinic receptor, ligand-gated ion channel, VAChT vesicular ACh transporter
Functions
Neurotransmitter

Vasodilating effect
Bronchial tree

Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides,2020



The epithelial cholinergic system of the airways, 2008
Scenario of a local auto-/paracrine role of epithelial ACh in regulating various aspects on the innate mucosal defence mechanisms. Pro-proliferative effects both on the epithelium and on subepithelial fibroblasts may occur in parallel
Vestibular labyrinth

Alpha-9 nicotinic acetylcholine receptor immunoreactivity in the rodent vestibular labyrinth, 2005
Fallopian tubes



The ignored structure for female fertility: fallopian tube cilia, 2024
Brain Cholinergic Pathways

Acetylcholine in mind: a neurotransmitter correlate of consciousness?, 1999

from Nancy J. Woolf, Ph.D site
Acetylcholine Imbalance in Early Memory Loss
Endothelium. 2000;7(2):83-92.
Endothelial cell calcium mobilization to acetylcholine is attenuated in copper-deficient rats.
Schuschke DA, Falcone JC, Saari JT, Fleming JT, Percival SS, Young SA, Pass JM, Miller FN.
Center for Applied Microcirculatory Research, University of Louisville School of Medicine, KY 40292, USA. DASCHU01@GWISE.LOUISVILLE.EDU
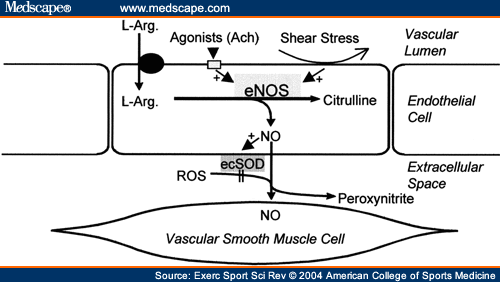
Dietary copper deficiency significantly attenuates nitric oxide (NO)-mediated vascular smooth muscle relaxation and vasodilation. There is evidence for both increased inactivation of the NO radical by superoxide anion, and oxidative damage to the endothelium where NO is produced. The current study was designed to examine the NO synthetic pathway in the endothelium during copper deficiency. Male weanling rats were fed a copper-adequate (CuA, 6.4 mg Cu/kg diet) or copper-deficient (CuD, 0.4 mg Cu/kg diet) diet for four weeks. Cremasteric arterioles (approximately 100 microm diameter) were isolated and used for the experiments. Western blot analysis of the arteriole endothelial nitric oxide synthase (eNOS) concentration did not show a difference between dietary groups. Acetylcholine (Ach)-induced vasodilation was significantly reduced in the CuD group both before and after pretreatment with the eNOS substrate L-arginine. Endothelial intracellular calcium ([Ca2+]i) stimulated by 10(-6) M Ach was significantly inhibited in the arterioles from CuD rats. Coincident with the inhibition of [Ca2+]i and vasodilation was a depression of vascular Cu/Zn-SOD activity and an increase in plasma peroxynitrite activity. These data suggest that endothelial Ca2+ signaling and agonist-stimulated NO-mediated vascular dilation are likely reduced by increased oxidative damage in copper-deficient rats.
Behav Brain Res. 2010 Feb 16. [Epub ahead of print]
The cholinergic system, nerve growth factor and the cytoskeleton., 2010
Niewiadomska G, Mietelska-Porowska A, Mazurkiewicz M.
Laboratory of Preclinical Studies in Neurodegenerative Diseases, Department of Neurophysiology, Nencki Institute, Warsaw, Poland.
Cholinergic neurons of the basal forebrain provide the major cholinergic innervation to the cortex and hippocampus, and play a key role in memory and attentional processes. Dysfunction of basal forebrain cholinergic neurons (BFCN) is a cardinal feature of Alzheimer's disease (AD) and correlates with cognitive decline. Survival of BFCN neurons depends upon binding of nerve growth factor (NGF), which is synthesized and secreted by cells in the cortex and hippocampus, with high-affinity (TrkA) and low-affinity (p75(NTR)) neurotrophin receptors produced within BFCN neurons. NGF released from target cells activates TrkA on axon terminals and triggers activation of PI3K/Akt, MEK/ERK, and PLCgamma signaling pathways. The signal then travels retrogradely along axon to cell body to promote neuronal survival. However, the nature of the retrograde signal remains mysterious. p75(NTR) receptors could mediate fundamentally different signaling pathway leading to apoptic cell death. Dysfunction of NGF and its receptors has been suggested to underlie the selective degeneration of the BFCN in end stage Alzheimer disease. In this regard, NGF, the founding member of the neurotrophin family, has generated great interest as a potential target for the treatment of AD. This review focuses on NGF-cholinergic dependency, NGF/receptor binding, signal transduction, retrograde transport, regulation of specific cellular endpoints, and the potential involvement of cytoskeleton dysfunction in defected NGF signaling. Copyright © 2010 Elsevier B.V. All rights reserved.