PTH and Gastrin
Evaluation of serum parathyroid hormone-related peptide in hyperthyroid patients, 2010
Abstract
Background Hypercalcemia occurs in 10–20% of patients with hyperthyroidism, but its pathogenesis is still unclear.
Aim To evaluate changes in parathyroid hormone-related peptide (PTH-rP) concentration in hyperthyroid patients compared with healthy controls.
Methods We studied PTH-rP, parathormone (PTH) and ionized calcium levels in 153 hyperthyroid patients, and 89 control subjects. These variables were revaluated after attainment of euthyroidism with the antithyroid drug carbimazole for 6 months in a subgroup of 47 patients.
Results Pretreatment PTH-rP and ionized calcium level were significantly higher in hyperthyroid patients than in controls, whereas an opposite trend occurred for PTH. All parameters normalized after carbimazole therapy.
Conclusion Untreated hyperthyroid patients exhibited a significant elevation in serum ionized calcium and PTH-rP and a significant reduction in serum PTH levels when compared with healthy controls. Our data favoured the hypothesis of a direct involvement of PTH-rP in the pathogenesis of hypercalcemia in hyperthyroid patients.

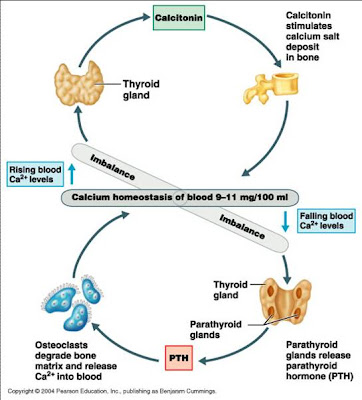
The cells of the parathyroid glands have surface G-protein-coupled receptors that bind Ca2+ (the same type of receptor is found on the calcitonin-secreting cells of the thyroid and on the calcium absorbing cells of the kidneys). Binding of Ca2+ to this receptor depresses the secretion of PTH and thus leads to a lowering of the concentration of Ca2+ in the blood. Two classes of inherited disorders involving mutant genes encoding the Ca2+ receptor occur:

defective PTH release can be asssociated to insuline defective release?

Calcium and phosphorus metabolism disturbances after renal transplantation.

Serum parathyroid hormone concentrations in senile dementia (Alzheimer's disease).
Shore D, Wills MR, Savory J, Wyatt RJ.
The accumulation of aluminum in the cerebral cortex has been implicated as a factor in the pathogenesis of Alzheimer type senile dementia (SD) and in the dialysis dementia found in patients with chronic renal failure on long-term intermittent hemodialysis treatment. In animal studies, parathyroid hormone (PTH) produces increased absorption of aluminum from the gastrointestinal tract and elevations of aluminum in the cerebral cortex. It has been proposed that PTH elevations may increase tissue aluminum loads in patients with senile dementia. The present study was undertaken to investigate the status of circulating PTH in patients with SD and age/sex matched controls. No significant differences were found between these groups. Elevated PTH (when it did occur) seemed to be related to the degree of renal impairment rather than dementia. Differences in the distribution of aluminum in patients with dialysis dementia and SD are discussed.
High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer's disease. 1998
Biosynthesis and function of all-trans- and 9-cis-retinoic acid in parathyroid cells. 1996
Chicken parathyroid hormone gene expression in response to gastrin, omeprazole, ergocalciferol, and restricted food intake, 1997
- Abstract
Treatment with omeprazole, a long-acting proton pump inhibitor of acid secretion, induces hypergastrinemia. In chickens, omeprazole induces growth not only of the acid-producing mucosa (probably reflecting the trophic action of gastrin), but also of the parathyroid glands (hypertrophy + hyperplasia), while suppressing bone density and body weight gain without affecting blood calcium. The first part of the present study was concerned with the effect of omeprazole, ergocalciferol (vitamin D2), and restricted food intake on the gene expression of parathyroid hormone (PTH) in the parathyroid glands of the chicken. Chickens were treated with omeprazole (400 micromol/kg/day, I.M.), food restriction, omeprazole + food restriction, ergocalciferol (250 000 IU/kg/day, S.C.), or ergocalciferol + omeprazole for 5 weeks. The weight gain of the chickens was monitored, and the weights of the parathyroid glands and femurs were determined at sacrifice. PTH mRNA in the parathyroid glands was analyzed by Northern blot. The second part of the study examined the effect of 3 weeks of continuous gastrin infusion (chicken gastrin 20-36, 5 nmol/kg/hour, S.C.) on the expression of PTH mRNA in the parathyroid glands. Omeprazole reduced the body weight and femur density (ash weight per volume) while greatly increasing the weight of the parathyroid glands and the PTH gene expression. Food restriction alone and ergocalciferol alone (at a dose that raised blood Ca2+) were without effect, but food restriction greatly enhanced the omeprazole-evoked increase in parathyroid gland weight and PTH gene expression. Gastrin increased the weight of the parathyroid glands and reproduced the effect of omeprazole on PTH gene expression. Hence, it seems likely that the effect of omeprazole reflects the ensuing hypergastrinemia.
Drugs increasing PTH release

Endocrine functions of bone in mineral metabolism regulation 2008
fulltext
PTH targets
Regulation of the PT cell Na+-Pi cotransporter Npt2a by PTH
Changes in plasma electrolytes during acclimatization at high altitude. 1996
Khan DA, Aslam M, Khan ZU.
J Pak Med Assoc. 1996 Jun;46(6):128-31.
The effects on plasma electrolytes and related hormones were determined in non-acclimatized low lander males, exposed for 96 hours to an altitude of 4424 meters. Twenty healthy soldiers aged 18-34 years travelled by road from an altitude of 2303 meters to 4424 meters over a period of 10 hours. Plasma sodium levels (142.09 +/- 1.14 mmol/1) and aldosterone (16.61 +/- 5.70 ng/ml) decreased to 139.69 mmol/1 and 11.6 +/- 4.60 ug/ml respectively after 96 hours of acute exposure to high altitude (p < 0.05). The plasma potassium and chloride levels did not show significant change, while, plasma HCO3 decreased gradually from 21.06 +/- 1.38 mmol/1 to 18.55 +/- 0.82 mmol/1 after 96 hours exposure to this altitude (p < 0.01). The plasma ionized calcium and plasma phosphate concentration decreased from 1.32 +/- 0.11 mmol/1 and 1.58 +/- 1.3 mmol/1 to 1.20 +/- 0.05 mmol/1 and 1.47 +/- 0.99 mmol/1 respectively (p < 0.05). Plasma parathyroid hormone (PTH) level increased from 4.54 +/- 2.1 ng/ml to 11.19 +/- 4.31 ng/ml after 48 hours with subsequent decline to 2.52 +/- 1.7 ng/ml after 96 hours exposure to high altitude. It may be concluded that the process of acclimatization to sudden exposure to high altitude is characterised by fall in plasma aldosterone and PTH with subsequent decrease of related electrolytes.
PTH and fgf-23 overexpression
PTH receptors
Identification of a retinoic acid-inducible element in the murine PTH/PTHrP (parathyroid hormone/parathyroid hormone-related peptide) receptor gene. 1999
PTH retinoic
cAMP+and+atrial+fibrillation
PTH and PSA
pth+and+psa