DEFINITION
O-6-methylguanine-DNA methyltransferase (MGMT) is a protein composed by 238 AA, that in humans is encoded by the MGMT gene.
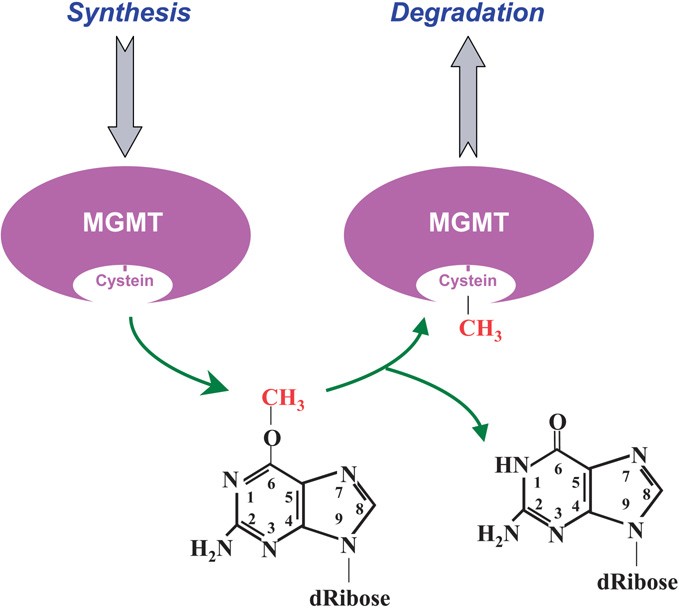


It is already known as Methylated-DNA-protein-cysteine methyltransferase or O-6-methylguanine-DNA-alkyltransferase (AGT or AGAT) and it is localized on the chromosome 10q26 position.
The MGMT protein is involved in the cellular defense against the biological effects of O6-methylguanine (O6-MeG) in DNA.
O6-MeG is a derivative of the nucleobase guanine in which a methyl group is attached to the oxygen atom. It base-pairs to thymine rather than cytidine. The MGMT protein is not a true enzyme since it accepts the alkyl group from the lesion in a stoichiometric reaction and it is then degraded by the proteasome. Thus the active enzyme is not regenerated after its alkilation. The methyl-acceptor residue in the protein is a cysteine.
MGMT protects human cells from the mutagenic effects of alkylating agents (e.g.: Nitrosourea, Carmustibe, Temozolomide) leading to O6-alkylguanine residues in the nuclear DNA.
O(6)-alkyl-guanine is the major carcinogenic lesion in DNA induced by alkylating mutagens. This DNA adduct is removed by the repair action of the MGMT protein.
Guanine is physiologically associated to Cytidine, but in case of alkylation on O-6 (e.g.: methylation), guanine will be base-pared with Thymine, resulting in a transition G>A after the next DNA replication if the mismatch is not repaired.

Demethylation mechanism of the MGMT Protein

When this type of alkylation happened, the MGMT protein transfers the alkylation from the guanine to its own structure. This reaction is stoichiometric, so one MGMT is required to remove one alkyl group. MGMT will be then tagged by Ubiquitin to be oriented to the proteasome for degradation.

THE GENE and TRASCRIPTION
The MGMT gene is composed of 5 exons separated by long introns (the genomic sequence is spread on more than 300kb)
The mRNA encodes for a 238 AA polypeptide . The mRNA sequence is 1265 bp long. Three others mRNA are documented on ENSEMBL but none of them are translated into protein.
CHEMICAL STRUCTURE AND IMAGES
The MGMT protein has a molecular weight of 25 kDa.
Sequence:
MLGQPAPLERFASRRPQVLAVRTVCDLVLGKMDKDCEMKRTTLDSPLGKLELSGCEQGLHEIKLLGKGTSAADA
VEVPAPAAVLGGPEPLMQCTAWLNAYFHQPEAIEEFPVPALHHPVFQQESFTRQVLWKLLKVVKFGEVISYQQ
LAALAGNPKAARAVGGAMRGNPVPILIPCHRVVCSSGAVGNYSGGLAVKEWLLAHEGHRLGKPGLGGSSGLAG
AWLKGAGATSGSPPAGRN
PROTEIN AMINOACIDS PERCENTAGE

3D STRUCTURE AND DOMAINS
The MGMT protein is composed of 2 main domains which are the methyl transferase domain, and the DNA binding domain.


P1= the methyl transferase domain P2= the DNA binding domain.

3D representation of the secondary structure of the MGMT protein
Ipotetical post translation modification

Only two sites have been associated with a functional consequence. The phosphorylation at the tyrosine 114 is associated with regulation of the protein activity, and the phosphorylation of the serine 201 confers a resistance to protease.
Up to date, there are no direct evidence of any post-translational modification of MGMT, nor it is known any particular mechanism involved in its degradation.

Cristal structure of the MGMT protein binding DNA; the O6 meg residue is totally buried into the active pocket of the enzyme;
Above: color at the surface represents the electrostatic potential of the MGMT protein;
positive charge (blue) , negative charge (red) neutral charge (white).
Below: heatmap at the surface represents the amino acid accessibility of the MGMT protein;
buried-less accessible amino acids (blue); exposed-more accessible amino acids (yellow-red).

LITERATURE
Esteller et al. (Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents, 2000) analyzed the MGMT promoter in tumor DNA by a methylation-specific PCR assay to determine whether methylation of the MGMT promoter is related to the responsiveness of gliomas to alkylating agents. The MGMT promoter was methylated in gliomas from 19 of 47 patients (40%). This finding was associated with regression of the tumor and prolonged overall and disease-free survival. It was an independent and stronger prognostic factor than age, stage, tumor grade, or performance status. The authors concluded that methylation of the MGMT promoter in gliomas is a useful predictor of the responsiveness of the tumors to alkylating agents.


In an evaluation of combined radiotherapy and Temozolomide (TMZ), an alkylating agent used to treat glioblastoma patients), for newly diagnosed glioblastoma (Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide, 2004), found that methylation of the MGMT promoter in the tumor was associated with longer survival.
Stupp et al. (Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma, 2005) showed that the addition of temozolomide to radiotherapy for newly diagnosed glioblastoma resulted in a clinically meaningful and statistically significant survival benefit with minimal additional toxicity.

Hegi et al. (MGMT gene silencing and benefit from temozolomide in glioblastoma, 2005) studied methylation of the MGMT promoter in newly diagnosed glioblastomas and found that patients with a methylated MGMT promoter in the glioblastoma benefited from temozolomide, whereas those who did not have a methylated MGMT promoter did not have such a benefit.

Testicular germ cell tumors are classified into 2 major histologic subgroups, seminomas and nonseminomas. In a series of 70 testicular germ cell tumors, Smith-Sorensen et al. (Frequent promoter hypermethylation of the O6-Methylguanine-DNA Methyltransferase (MGMT) gene in testicular cancer, 2002) analyzed for methylation of CpG sites in the MGMT gene promoter and in exon 1-alpha of the cyclin-dependent kinase inhibitor 2A gene (CDKN2A). None of 55 tumors showed methylation of CDKN2A. On the other hand, high frequencies of MGMT promoter methylation and allelic imbalances at 10q markers were found in 32 of 69 (46%) and 50 of 70 (71%) tumors, respectively. A significantly higher methylation frequency was found in 24 of 35 nonseminomas (69%) compared to 8 of 33 seminomas (24%). Immunohistochemical analysis of the MGMT protein in a subgroup of the testicular tumors supported the hypothesis of gene silencing being the functional consequence of the promoter methylation. The data suggested that inactivation of MGMT contributes to the development of nonseminomatous testicular cancer.
By examining gene expression profiles, Fry et al. (Genomic predictors of interindividual differences in response to DNA damaging agents, 2008) showed that elevated MGMT expression was associated with reduced sensitivity to MNNG, a DNA alkylating agent. Analysis of genes that were differentially expressed between cell lines with the highest and lowest MNNG sensitivities integrated MGMT into a protein network related to human cancer and tumorigenesis.
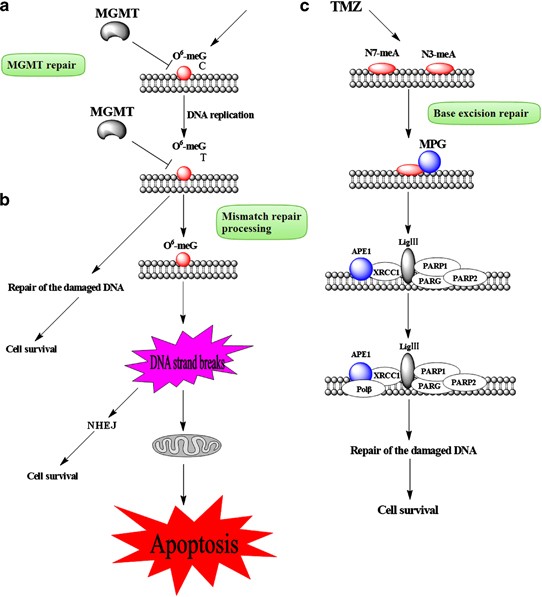
The Cancer Genome Atlas Research Network reported the interim integrative analysis of DNA copy number, gene expression, and DNA methylation aberrations in 206 glioblastomas and nucleotide sequence alterations in 91 of the 206 glioblastomas. A link was found between MGMT promoter methylation and hypermutator phenotype consequent to mismatch repair deficiency in treated glioblastomas. The methylation status of MGMT predicts sensitivity to temozolomide (TMZ), an alkylating agent used to treat glioblastoma patients. In those patients who also have mutation in the mismatch repair pathway, treatment with an alkylating agent was associated with characteristic C-G and A-T transitions in non-CpG sites, raising the possibility that patients who initially respond to treatment with alkylating agents may evolve not only treatment resistance but also a mismatch repair-defective hypermutator phenotype.
TEMOZOLOMIDE (TMZ)
It is used for the treatment of Grade IV astrocytoma, an aggressive brain tumor, also known as glioblastoma multiforme (OMIM database ) — as well as for treating melanoma (OMIM database ), a form of skin cancer.
Methylation of the gene's promoter may play a significant role in carcinogenesis. As described above, in patients with glioblastoma multiforme, a severe type of brain tumor, the methylation state of the MGMT gene determined whether tumor cells would be responsive to temozolomide; if the promoter was methylated, temozolomide was more effective (MGMT gene silencing and benefit from temozolomide in glioblastoma, 2005).
This drug is well tolerated and is able to diffuse in the whole body (including brain) due to its low molecular weight. Its peak plasma concentration is at 1h and its half life is estimated at 1.8h.
The agent was developed by Malcolm Stevens (Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials, 1997) and his team at Aston University in Birmingham.
The therapeutic benefit of temozolomide depends on its ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues. This methylation damages the DNA and triggers the death of tumor cells. However, some tumor cells are able to repair this type of DNA damage, and therefore diminish the therapeutic efficacy of temozolomide, by expressing the MGMT protein. In some tumors, epigenetic silencing of the MGMT gene prevents the synthesis of this enzyme, and as a consequence such tumors are more sensitive (MGMT gene silencing and benefit from temozolomide in glioblastoma, 2005) to killing by temozolomide.Conversely, the presence of MGMT protein in brain tumors predicts poor response to temozolomide and these patients receive little benefit from chemotherapy with temozolomide.

STRUCTURE AND MECHANISM
Temozolomide (TMZ) is an imidazotetrazine derivative of the alkylating agent dacarbazine. It undergoes rapid chemical conversion in the systemic circulation at physiological pH to the active compound, MTIC (monomethyl triazeno imidazole carboxamide). Temozolomide exhibits schedule-dependent antineoplastic activity by interfering with DNA replication. Temozolomide has demonstrated activity against recurrent glioma. In a recent randomized trial, concomitant and adjuvant temozolomide chemotherapy with radiation significantly improves, from 12.1 months to 14.6 months, progression free survival and overall survival in glioblastoma multiforme patients.

Side-effects
The most common non-hematological adverse effects associated with temozolomide - nausea and vomiting - are either self-limiting or readily controlled with standard antiemetic therapy. These effects are usually mild to moderate (grade 1 to 2). The incidence of severe nausea and vomiting is around 4% each. Patients who have pre-existing or a history of severe vomiting may require antiemetic therapy before initiating temozolomide treatment. Temozolomide should be administered in the fasting state, at least one hour before a meal. (Capsules must not be opened or chewed, but swallowed whole with a glass of water). Antiemetic therapy may be administered prior to, or following, administration of temozolomide. Temozolomide is contraindicated in patients with hypersensitivity to its components or to dacarbazine. The use of temozolomide is not recommended in patients with severe myelosuppression.
The current standard treatment for the Glioblastoma multiforme (GBM) includes chemotherapy with the DNA-alkylating agent temozolomide concomitant with surgical resection and/or irradiation. However, a number of cases are resistant to temozolomide-induced DNA damage due to elevated expression of the MGMT protein. The upregulation of both MGMT and STAT3 was accompanied with acquisition of temozolomide resistance in the GBM cell line U87. Inactivation of STAT3 by inhibitor or short hairpin RNA (shRNA) downregulated MGMT expression in GBM cell lines. MGMT upregulation was not observed by the treatment of interleukin
IL-6 which is a strong activator of STAT3. Contrarily, forced expressed MGMT could be downregulated by STAT3 inhibitor which was partially rescued by the proteasome inhibitor, MG132, suggesting the STAT3-mediated posttranscriptional regulation of the protein levels of MGMT. Immunohistochemical analysis of 44 malignant glioma specimens showed significant positive correlation between expression levels of MGMT and phosphorylated STAT3 (p-STAT3; P < 0.001, r = 0.58). Importantly, the levels of both MGMT and p-STAT3 were increased in the recurrence compared with the primary lesion in paired identical tumors of 12 cases. In conclusion STAT3 inhibitor or STAT3 knockdown potentiated temozolomide efficacy in temozolomide-resistant GBM cell lines. Therefore, STAT3 inhibitor might be one of the candidate reagents for combination therapy with temozolomide for patients with temozolomide-resistant GBM (STAT3 Inhibition Overcomes Temozolomide Resistance in Glioblastoma by Downregulating MGMT Expression, 2012) .
MGMT REGULATION AND LOCALIZATION
Reduction of MGMT activity was due to a decline of both MGMT mRNA and protein levels. Protein expression is not significantly correlated with promoter methylation however the methylation is thought to be the main process of regulation.
Most of the immunostaining data, including human biopsy tissues, showing that human MGMT is a nuclear protein (Intracellular localization of human DNA repair enzyme methylguanine-DNA methyltransferase by antibodies and its importance, 1992). However, there are reports on its cytosolic appearance (Artificial control of nuclear translocation of DNA repair methyltransferase, 1994). This could be due to the cell lines used, which might be defective in the mechanism of nuclear localization of human MGMT, antibody specificity or experimental conditions. Therefore, understanding the mechanisms behind the nuclear localization of the protein may provide information for the above unusual observations and, importantly, an insight into how the protein functions in vivo.
TMZ XENOGRAFT SENSITIVITY
Few xenograft experiments have been performed. An article by Carter et al (Both extraneuronal monoamine transporter and O(6)-methylguanine-DNA methyltransferase expression influence the antitumor efficacy of 2-chloroethyl-3-sarcosinamide- 1-nitrosourea in human tumor xenografts, 2001) published in proceeding of American Cancer Research in 2009 described response in xenografted mice.
TMZ survival evaluation relative to MGMT methylation and protein expression. Mice with established orthotopic xenografts were randomized and then treated with placebo or TMZ orally. Survival curves are shown below.

CLINICAL TRIALS: USE IN CANCER
Temozolomide is approved to treat the following types of brain tumors in adults:
- Anaplastic astrocytoma
- Glioblastoma multiforme
Temozolomide is also being studied in the treatment of other solid tumors (Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase, 2001) including colorectal cancers.

OTHER USES
MGMT has also been shown to be a useful tool increasing gene therapy efficiency (Erythroid-specific Human Factor IX Delivery From In Vivo Selected Hematopoietic Stem Cells Following Nonmyeloablative Conditioning in Hemophilia B Mice, 2008). By using a two component vector consisting of a transgene of interest and MGMT, in vivo drug selection can be utilized to select for successfully transduced cells.
When overexpressed in stem cells, MGMT serves as a drug-selection gene for gene therapy and protects normal tissues from the toxic effects of chemotherapy.