What is Aspartame?

Aspartame (APM) is an artificial, non-saccharide, low-calorie sweetener used as a sugar substitute in foods and beverages.
- molecular formula: C14H18N2O5
- IUPAC name: N-(L-a-Aspartyl)-L-phenylalanine,1-metyl ester
- other names: E951
- Nutrasweet
It is composed of two aminoacids: aspartic acid and phenylalanine; the carboxyl end of phenylalanine is esterified with methanol.

Even if it has the same calories of sucrose, its sweetening power is 200 times stronger: that's why it is used in small quantities.
The sweetness of aspartame lasts longer than sucrose, so it is often blended with other artificial sweeteners such as acesulfame-K to produce an overall taste more like sugar.
The allowable daily dose is 40mg/kg BW: regular consumers of products containing aspartame normally exceed this threshold.
At room temperature, aspartame is most stable at pH 4.3, where its half life is nearly 300 days.
Under strongly acidic or alkaline conditions, aspartame may generate methanol by hydrolysis.
Under more severe conditions, like elevated temperature or high pH, the peptide bonds are also hydrolyzed, resulting in the free amino acids.
For this reason it is preferably used in soft-drinks, having a pH between 3 and 5, where aspartame is reasonably stable.
Two approaches to synthesis are used:
- CHEMICAL SYNTHESIS: the two carboxyl groups of aspartic acid are joined into an anhydride, and the amino group is protected by a compound that will prevent further reactions of that group.
Phenylalanine is methylated and combined with the N-protected aspartic anhydride, then the blocking group is removed from aspartic acid by acid hydrolysis.
The drawback of this technique is that a byproduct, the bitter tasting β-form, is produced when the wrong carboxyl group from aspartic acid links to phenylalanine.
A process using an enzyme from Bacillus thermoproteolyticus to catalyze the condensation of the chemically altered amino acids will produce high yields without the β-form byproduct.
- NON-COMMERCIAL SYNTHESIS : it is a variant of the previous method, which uses unmodified aspartic acid, but produces low yields; for this reason it is not been scaled for industrial production.
(http://it.wikipedia.org/wiki/Aspartame)
Mechanism of action

Upon ingestion, aspartame breaks down into residual components, including aspartic acid, phenylalanine, methanol, in ratio of 4:5:1 by mass, and further break down products including formaldehyde and formic acid.
Methanol is gradually released in the small intestine when the methyl group of aspartame meets the enzyme Chymotrypsin.
Free methanol begins to form when a liquid is brought to temperatures exceeding 30°C.
Then methanol is converted into formaldehyde, which produces formic acid.
An EPA (Enviromental Protection Agency - USA) evaluation on methanol considers it a poison of accumulation, for its low rate of excretion when absorbed and many studies confirm this.


Phenylalanine and aspartic acid are parts of our physiological metabolism, if assumed with other amino acids, if not they become toxic too.
ASPARTAME AND PHENYLKETONURIA
High levels of the naturally - occurring essential amino acid phenylalanine are a health hazard to those born with Phenylketonuria (PKU), a rare inherited disease that prevents phenylalanine from being properly metabolized.
Since individuals with PKU must consider aspartame as an additional source of phenylalanine, foods containing aspartame sold in the United States must state "Phenylketonurics: Contains Phenylalanine" on their product labels.
(Aspartame...the inconvenient truth, by Mark Gold)
Discovery and approval
Aspartame was discovered in 1965 by James M. Schlatter, a chemist working for G.D. Searle & Co. , who synthesized aspartame as an intermediate step in generating gastrine, for use in assessing an anti-ulcer drug candidate.
He accidentally discovered its sweet taste licking his finger contaminated with aspartame.
From that moment to 1983 FDA strongly opposed the marketing of this sweetener.
Under Reagan's government, several political pressures silenced contrary opinions and aspartame was allowed for use in carbonated beverages, and for use in other beverages, baked goods, and confections in 1993.
In 1996, the FDA removed all restrictions from aspartame, allowing it to be used in all foods.
Several European Union countries approved aspartame in the 1980s, with EU-wide approval in 1994.
The European Commission Scientific Committee on Food reviewed subsequent safety studies and reaffirmed the approval in 2002.
The European Food Safety Authority reported in 2006 that the previously established Acceptable Daily Intake was appropriate, after reviewing yet another set of studies.
Aspartame has been found to be safe for human consumption by more than ninety countries worldwide, with FDA officials describing aspartame as "one of the most thoroughly tested and studied food additives the agency has ever approved".
Therefore, nowadays we can find it in many products, like jam for diabetics, diet drinks, sugar-free chewing-gum, breath mints, cereals, milk drinks, pharmaceutical drugs and supplements, yogurt, desserts, etc...

(Wikipedia; Report: dagli zuccheri raffinati, ai dolcificanti artificiali e poi naturali; Sweeteners and sugar alternatives in food technology Blackwell. pp. 86–951-4051-3434-8HYPERLINK Retrieved 26 July 2011.)
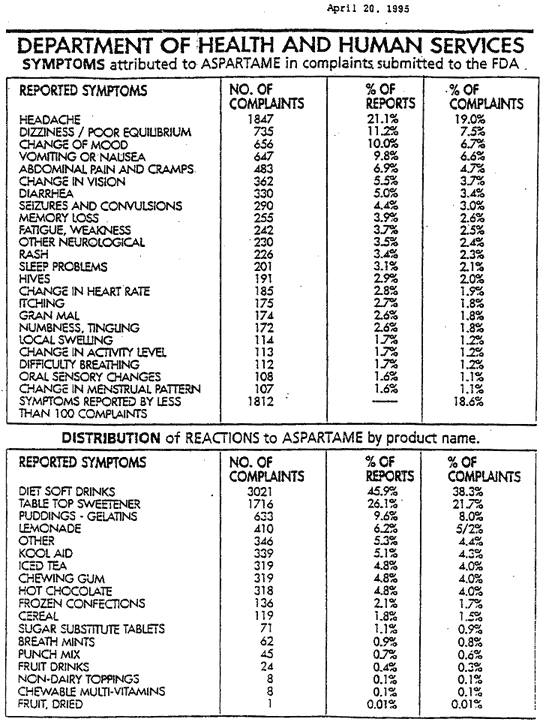
Aspartame side effects
The damages of aspartame are now recognized by most of the medical community.
They can occur gradually, can be immediate, or can be acute reactions.
We mention just some of them:
* Eye [see HUMAN VISUAL PATHWAY DAMAGE]
blindness in one or both eyes
decreased vision and/or other eye problems such as: blurring, bright flashes, squiggly lines, tunnel vision, decreased night vision
pain in one or both eyes
decreased tears
bulging eyes
* Ear
tinnitus - ringing or buzzing sound
severe intolerance of noise
marked hearing impairment
* Neurologic [see DAMAGES TO HYPOTHALAMUS]
epileptic seizures
headaches, migraines (some severe)
dizziness, unsteadiness
confusion, memory loss
severe drowsiness and sleepiness
paresthesia or numbness of the limbs
severe slurring of speech
severe hyperactivity and restless legs
atypical facial pain
severe tremors
* Psychological/Psychiatric
severe depression
irritability
anxiety
personality changes
insomnia
phobias
* Chest
palpitations, tachycardia
shortness of breath
recent high blood pressure
* Gastrointestinal
nausea
diarrhea, sometimes with blood in stools
abdominal pain
pain when swallowing
* Skin and Allergies
itching without a rash
lip and mouth reactions
hives
aggravated respiratory allergies such as asthma
* Endocrine and Metabolic
loss of control of diabetes
menstrual changes
marked thinning or loss of hair
marked weight loss
gradual weight gain
aggravated low blood sugar (hypoglycemia)
severe PMS
* Other
frequency of voiding and burning during urination
excessive thirst, fluid retention, leg swelling, and bloating
increased susceptibility to infection
(http://www.sweetpoison.com/aspartame-side-effects.html)
(http://www.whale.to/b/aspartame.html)
HUMAN VISUAL PATHWAY DAMAGE
When aspartame enters the upper portion of intestine, methanol released enters the blood stream and reaches the highly metabolic region of the optic nerve and retina, where partial atrophy can and does take place.
Methanol is particularly toxic to the optic nerve, leading to acute blindness.
The vision cannot do without oxygen and nutrition for more than ninety seconds without revealing some damage.
Total loss of vision can occour and there is no return.
In the very early stages in which is referred to as the "wet stager", treatment can be given and will reserve the destructive pathology to the optic nerve and retina.
The physician should understand the chemical ongoing process.
(http://rense.com/general65/aspar.htm)

MRI on day 15 after methanol intoxication.
a) T2-weighted image showed high signal abnormalities in bilateral basal ganglia (arrows), frontal, and occipital subcortical white matter (arrowheads), consistent with oedematous change.
b) T2-weighted image showed oedematous change involving bilateral optic tracts and optic radiations (arrows).
High signal oedematous change was also noted in the optic disc of left eye (arrowheads).
c) T1-weighted image showed slightly high signal component in bilateral basal ganglia, indicating the haemorrhage (arrows).
d) T1-weighted image with gadolinium administration showed marginal enhancement in bilateral putamen, indicating breakdown of the blood−brain barrier.

Fundus photography 2 months after methanol intoxication showed optic atrophy with glaucomatous-like cupping of the optic discs, and narrow neuroretinal rim with 0.9 cup in (a) right eye and 0.7 cup in (b) left eye.
There was generalized narrowing of the retinal arteries.
(Sharpe JA, Hostovsky M, Bilbao JM & Rewcastle NB. Methanol optic neuropathy: a histopathological study. Neurology 1982; 32: 1093−1100. | PubMed)
(Baumbach GL, Cancilla PA, Martin-Amat G, Tephly TR, McMartin KE & Makar AB et al.. Methyl alcohol poisoning IV: alterations of the morphological findings of the retina and optic nerve. Arch Ophthalmol 1977; 95: 1859−1865. | PubMed)
(Kuteifan K, Oesterle H, Tajahmady, Gutbub AM & Laplatte G. Necrosis and hemorrhage of the putamen in methanol poisoning shown on MRI. Neuroradiology 1998)
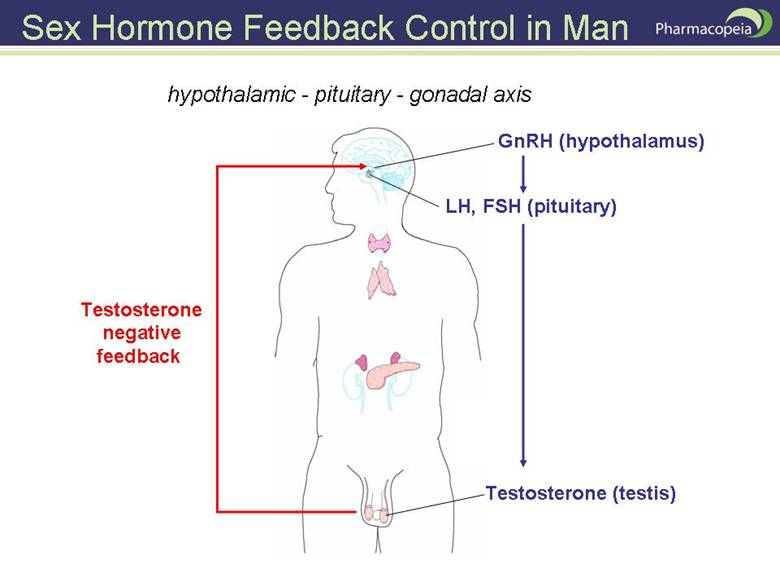
DAMAGES TO HYPOTHALAMUS
The hypothalamus produces gonadotropin-releasing hormone ( GRH ), which goes down the stalk between the hypothalamus and pituitary and causes the pituitary then to produce gonadotropins.
The ganglia goes to the testicles and causes them to produce testosterone.
When you're causing hypothalmic destruction with neuroexcitotoxins like aspartame, you're suppressing the formation of male hormone without which there is no sexual drive or pleasure for either.
In original studies aspartame triggered atrophied testes and testicular tumors.
It happens in this way: aspartame destroys the myelin sheaths and the nerves and sheaths try to regenerate but now the signals can be crossed.
So the pleasure receptor of the penis sends the signals but it arrives at the brain at a wrong receptor and it is not recognized as pleasure.
The methyl alcohol type of poisoning from aspartame is the foremost known cause of degeneration of the sheaths and the ganglia.
The excitotory area of the cerebral cortex which allows men to be excited, interested and pleasured by sex, atrophies when the testosterone is suppressed.
Moreoever, you have an independent neurotoxin generated by the isolated phenylalanine.
Anytime you have a neurotoxin making the brain sick, sexual pleasure is obliterated because the brain is the most important sexual organ.
Serotonin and dopamine levels are suppressed.
(Aspartame damages Hypothalamus,James Bowen, M.D. 1720 North Watts, Portland, Oregon 97217, 22 Feb 2000)

IS THERE A LINK BETWEEN ASPARTAME AND CANCER?
In the past two decades brain tumor rates have risen in several industrialized countries, including the United States.
This increase is certainly due to an improvement of the diagnostic technology but there are other environmental factors linked to brain tumors.
A promising candidate was aspartame: in order to test its mutagenic potential, in the early 1980's several studies were conducted on rodents, which did not reveal toxic nor carcinogenic effects of the compound.
However, in 2005 a long term study conducted in _European Ramazzini Foundation (ERF)_ in Bologna, provided the first experimental demonstration that APM, when administrated in feed at various doses to Sprague-Dawley rats for the lifespan, is a multi-potent carcinogenic agent.
"A second study, published by the ERF in 2007, demonstrated that when lifespan exposure to APM begins during fetal life, its carcinogenic effects are increased.
In particular there was an increased incidence of lymphomas and leukaemias in male and female rats, and of breast carcinoma in female rats.
Although the result of these studies have been contested by the manufacturers of APM, concerns about the safety of aspartame continues to grow among citizens and remains on the agenda of the agencies responsible for overseeing food safety.
(Journal of Neuropathology and Experimental Neurology(1996), 55(11):1115-1123 | PubMed)
(La Rivista di Scienza dell’Alimentazione, numero 4, ottobre-dicembre 2008, anno 37)
(Morando Soffritti et al., European Journal of Oncology, Apartame induces lymphomas and leukemias in rats, vol. 10, n. 2, July 2005)
(National Cancer Institute Website)
(http://www.cancer.org/cancer/cancercauses/othercarcinogens/athome/aspartame)

Conclusions
Considering that the results of experimental tests conducted on rodents are highly predictive of cancerogenic risks to humans (as acknowledged by the International Agency for Research on Cancer - IARC - of World Health Organization), the results of these studies impose an urgent review of the acceptable intake levels for aspartame by the authorities responsible.
In addition, it has to be kept in mind that there is no threshold below which a carginogen can be considered safe for humans, and that the quality assurance of foods has always been a primary goal of legislators.
For example in the USA the Delaney amendment says: “It is not allowed to use any food additive for human nutrition in any quantity, for which appropriate trials have proved to be cancerogenic, if administrated to humans or animals”.
We need appropriate scientific knowledge in the context of artificial sweeteners as soon as possible, especially considering the increasing diffusion of low-calories products: according to a study published on the New York Times (May, 15th , 2005), 2225 new sugar-free consumer goods were introducted on the u.s. Market only in 2005, representing the 11% of all the new foodstuffs entered that year.
Considering these numbers, a re-evaluation of the effects of this substance is necessary, especially for more exposed categories, like children and fertile women: for this reason the European Ramazzini Foundation (EFR) has been carrying on for a long time a research program to evaluate the potential carcenogenic risks of the most common sweeteners.