We have evaluated the uses of the gold compounds in therapy, not only in our days, but also in the past.
Medical and therapeutic value of gold has been recognized thousands of years ago, but its rational use in medicine has not begun until the early 1920s. Gold is one of the oldest and most effective drugs in the treatment of rheumatoid arthritis. Nowadays, gold compounds constitute a family of very promising experimental agents for cancer treatment.

Chemical structures of gold(I) compounds for rheumatoid arthritis treatment.
Aurothiomalate, Aurothioglucose and Auranofin are the first gold compounds used in modern therapy and they are gold thiolates. These are similar to the new ones used in breast cancer therapy, also if these are dithiocarbamate complexes.

R2 and R3 in the image are either Cl or Br.
The gold porphyrin complexes used in colon cancer therapy is different from the other ones. Infact there is a typical aromatic structure with an Au (III) bound with the four amino groups.

Indications of Use
- Inflammatory diseases
- Rheumatoid arthritis
- Colon cancer
- Breast cancer
- Autoimmunitary disease
Pharmacokinetics
The gold compounds are normally injected in the patient, but the auronafin have an oral administration. The gold from injectable gold compounds is widely distributed throughout the reticulo-endothelial system, particularly in the phagocytic cells of the liver, bone marrow, lymph nodes, spleen, and also in the synovium (this is important for RA treatment). Deposition in the skin occurs and it has been stated that there is a quantitative correlation between the amount of gold in the dermis and the total dose given. The injectable gold-thiol compounds, contains Au(I) and also binds predominantly to albumin. After oral administration to human volunteers of 195Au- labelled Auranofin, approximately 25% of the administered dose is detected in plasma, peak concentrations of 6–9 µg/100 mL being reached within 1–2 h. The plasma half life is in the order of 15–25 days with almost total body elimination after 55–80 days. Gold has also been detected in the milk of lactating mothers and in the serum and red blood cells of nursing infants. The presence of gold in the infant indicates that it was originally in the maternal milk, presented to the infant, as an orally absorbable form, possibly that bound to albumin or macroglobulins.
The pharmacodinamic of the compounds is various, in relations with the actions in different patology.
Clinical Pharmacology of Gold, 2008
Molecular Mechanism
1. Modulation of immune system
Gold unfolds its therapeutic efficacy through various influences on the immune system. Gold alters antigen- processing and reduces cythokine expression of macrophages. Additionally, gold reduces adhesion molecules, antibody production and inhibits proteolytic enzymes.The gold-thiol drugs can inactivate the first component of complement (C1), and inhibit lysosomal hydrolytic enzymes such as acid phosphatases. The effect of gold compounds in vivo and in vitro on various immune responses, has been described by many groups. Gold sodium thiomalate treatment reduced all classes of immunoglobulin and also serum rheumatoid factor. The gold salts inhibited phytohaemagglutinin (PHA)-induced thymidine incorporation and gamma-IF production by peripheral blood mononuclear cells, as well as IL-2-induced proliferation of PHA-blasts. This made gold compound usefull for inflammatory disease treatment.
Unique properties of auranofin as a potential anti-rheumatic drug, 1986
Gold and modulation of the immune respons, 1979
2. Inhibition of NF-KB system
NF- B is a transcription factor implicated in the expression of many inflammatory genes. NF- B is activated by signal-induced κ κ phosphorylation and subsequent degradation of inhibitory I B (inhibitory protein that dissociates from NF- B) proteins, and a κ κ multisubunit I B kinase (IKK) has been identified previously.
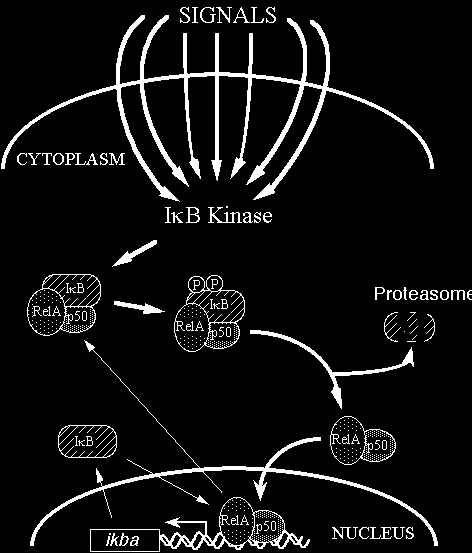
Nuclear factor-B is a transcription factor that plays a pivotal role in the expression of a wide range of genes involved in chronic κ inflammatory diseases, including TNF, IL-1, IL-6, IL-8, GM-CSF, inducible NO synthase, ICAM-1, E-selectin, and the MHC class I and II molecules. Macrophages have been recognized as playing important roles in the pathogenesis of RA, in that there is a relative abundance of macrophage-derived cytokines, such as TNF and IL-1, in rheumatoid synovium. Gold compounds (AM, AG e AF), comprised of elemental Au(I) and a sulfur-containing ligand, have been used for the treatment of RA, inducing improvement of clinical conditions in a majority of patients. The inhibitory effect of auranofin appeared by reducing mRNA level of IL-1 and TNF, suggesting that it blocks some common step in the signal pathways for the transcriptional activation of them. Gold compounds were able to suppress IKK activity when added directly to an in vitro kinase assay, a property shared with other thiol- binding metal ions such as zinc, copper, and mercury and thiol- reactive agents. Our data imply that IKK contains a metal-sensitive cysteine residue and is one of the major targets of gold and related metal compounds in the treatment of RA. Auranofin inhibits signal- induced activation of IKK. Our results that metals such as gold, zinc, and copper inhibit IKK activity in vitro and induction of IKK in LPS- stimulated macrophages suggest that a metal-sensitive thiol group exists in IKK complex and plays a critical role in the regulation of enzyme activity. The inhibitory effect of auranofin was associated with suppression of I B and I B degradation, as well as the κ α κ β suppression of IKK activation. In view of the critical role of NF- B in the transcriptional activation of both TNF and IL-1 genes, κ β these results indicate that auranofin inhibits expression of these cytokines by blocking LPS-induced activation of IKK, and thereby NF- B activation. κ These metal ions are commonly known to interact with sulfur- or nitrogen-containing groups of an enzyme such as thiol and imidazole groups . There is a sulfhydryl group in IKK that is critical for the enzyme activity, and the metal ions inhibit IKK activity by binding to this site. Structural data of IKK subunits reveal that cysteine residues are present in the kinase domain of IKK and IKK and some of α β them located at functionally important sites such as activation T loop and the catalytic site. Oxidation of this sulfhydryl group to disulfide, binding to metal ions, or blocking with thiol-groupreactive agents seems to inactivate the enzyme. This molecular mechanism made gold compounds excellent drugs for rheumatoid arthritis.
Molecular mechanisms of action of gold in treatment of rheumatoid arthritis, 2001
Thiol-reactive metal compounds inhibit NF-kappa B activation by blocking I kappa B kinase, 2000
3. Induction of mitochondrial apoptosis and cell cycle arrest in tumor cells
Gold (III) compounds have exhibited favorable antitumor properties both in vitro and in vivo. Gold (III) compounds exhibit strong cytotoxic activity in vitro against tumor cells and have tumor-inhibiting properties in vivo. However, currently, some available gold (III) compounds exhibit poor solubility or are effective only at relatively high doses. A series of gold (III) mesotetraarylporphyrins was recently syhnthetized and these are stable against demetallation in physiologic conditions. The porphyrin ligands in these compounds can stabilize the gold (III) center and carry the metal to their cellular targets. One of these new gold (III) compounds,gold 1a, exhibits 30-fold to 100-fold stronger cytotoxicity than the older drug such as cisplatin. Treatment with gold 1a induced marked morphologic changes, including cytoplasmic vacuoles and cellular fragmentation and nuclear shrinkage 24 hours and 48 hours after treatment. There was a significant increase in the percentage of apoptotic cells. Treatment with gold 1a consistently resulted in the expression of several markers of apoptosis, such as caspase 3, caspase 7, and poly(adp-ribose) polimerase (PARP) cleveage and in the release of mitocondrial citocrome c into the citosol Gold 1a Causes Cell Cycle Arrest in G0/G1 Phase resulting in the inhibition of cancer cell growth. addition, in vivo and in vitro binding assays showed that gold(III) porphyrin 1a acted on DNA

Western blot analysis indicated that gold 1a up-regulated the expression of p21, p27, and Bax proteins and decreased the expression of bcl-xl, whereas there was no change in the levels of bcl-2 protein. Levels of p53 protein began to rise 12 hours after treatment, reached the maximum level after 24 hours, and decreased thereafter . An increase in p27 protein level occurred at later intervals (48 hours and beyond). The bcl-xl level decreased after 24 hours but then recovered at 48 hours after treatment . These results indicate that gold 1a induces cell cycle arrest and apoptosis by regulating the expression of p53, p21, p27, and Bax. These results suggest that the inhibition of tumor growth is caused by an increase in apoptosis and a decrease in cell proliferation induced by gold 1a in vivo. The mechanisms responsible for gold (III) complex antitumor activity still are largely unknown. It is believed generally that the cytotoxic effects of metal complexes are the consequences of direct damage to nuclear DNA. However, some studies have demonstrated that gold (III) complex interactions with DNA are not as tight as platinum interactoins. Gold (III) compounds inhibit DNA and RNA synthesis; probable binding sites for gold(III) are N(1)/ N(7) atoms of adenosine, N(7) or C(6)O of guanosine, N(3) of cytidine, and N(3) of thymidine. This properties made gold(III) compound an anti- Colon cancer treatment.
Gold (III) porphyrin complexes induce apoptosis and cell cycle arrest and inhibit tumor growth in colon cancer, 2009
4. Gold as inhibitors of proteasome
Various proteasome inhibitors such as gold compounds, potently induce tumor cell apoptosis. Proteasome inhibitors can mechanistically work in several ways. One of the most common mechanisms requires binding to the proteasomal binding pockets, which then prevents substrate binding. After the observations that aberrant proteasome-dependent proteolysis might be associated with the pathophysiology of some malignancies, proteasome inhibitors were suggested as a novel class of anti- cancer drugs expecially in the breast cancer treatment. Increased proliferation of cancer cells is associated with higher rate of synthesis and degradation of many regulatory proteins.

In human breast cancer, proteasome has a critical role in maturation of P-glycoprotein, a membrane pump that, among others, promotes the efflux of chemotherapeutic drugs. By inhibiting the proteasome, P- glycoprotein levels in cancer cells membranes will decrease, allowing for accumulation of the used chemotherapeutic and consequently increasing the drug's effectiveness. The available data strongly suggest that the proteasome is one of the primary targets for gold(III) dithiocarbamates and that inhibition of the proteasomal activity by gold(III) dithiocarbamates is associated with apoptosis in cancer cells. Moreover these gold(III) dithiocarbamates stimulate production of ROS and they modify some mitochondrial functions, inactivate both cytosolic and mitochondrial thioredoxin reductase and interfere with ERK pathway inducing cancer cell death through both apoptotic and non-apoptotic mechanisms. During the past decades, breast cancer treatment has been rapidly evolved leading to substantial reduction in breast cancer mortality. New drugs have been synthesized and evaluated in these years; one class of drugs that is getting more attention and is showing promising results in the area of anti-cancer drug discovery is a class of gold- based complexes. Considerable interest in this area is encouraged by a variety of new gold complexes that have already been synthesized or are still waiting to be synthesized and evaluated. While developing new gold-based anti-cancer drugs, it is essential to design a drug that would target a specific biological site, resulting in reduced or no toxic side effects. For that reason, it is of paramount importance to better understand the coordination chemistry and molecular and biochemical mechanisms of gold complexes, which will also provide the new impetus for development of gold-based drugs. By following the interactions between gold complexes and various biological targets, much progress will be made in understanding the mode of action of gold complexes.
The tumor proteasome as a novel target for gold(III) complexes: implications for breast cancer therapy, 2009
5. Allosteric interaction between gold compound and MHC
Class II major histocompatibility complex proteins are essential for normal immune system function but also drive many autoimmune responses. They bind peptide antigens in endosomes and present them on the cell surface for recognition by CD4 T cells. Small molecules such as gold complexes could potentially block an autoimmune response by disrupting MHC-peptide interactions, probably with an allosteric interaction. This previously unknown allosteric mechanism may provide a basis for developing a new class of anti-autoimmune drugs.
Side-effects and toxicity
- eryptosis: gold stimulates Ca2+ entry into and subsequent suicidal death of erythrocytes. The suicidal death of erythrocytes, eryptosis, is characterized by cell shrinkage and cell membrane scrambling leading to phosphatidylserine exposure at the erythrocyte surface. Erythrocyte cell membrane scrambling is stimulated by increase of cytosolic Ca2+ concentration.
- Ipersensibility due to the antigernic properties of gold, this cause cutaneous rashes.
Gold stimulates Ca2+ entry into and subsequent suicidal death of erythrocytes, 2008
Conclusion
The therapeutic and beneficial effect of gold on human health have been known for centuries. Despite the considerable effort to develop new, efficient, and safe gold-based drugs, very few advances have been made. A significant amount of effort in medicinal gold chemistry involves the development of novel anti-cancer drugs and identification of their novel molecular targets.
Tiralongo Paolo, Paruzzo Luca