When we hurt ourselves, one of the first instinctive responses we have is to lick the injury. Also in dogs, cats, rodents and all primates we can find this behavior.

The first suggestion that saliva may have properties that aid wound healing come from the observation that oral mucosa heals faster than skin.
Then, with the study of the composition of saliva, several enzymes and molecules were found and described to have many functions in wound healing: antibacterial, antiviral, promoting healing and tissue growth.

Between these, there is another effect, discovered in recent years: an analgesic effect due to the pentapeptide opiorphin, an opioid present in saliva. This has revealed to be a potent analgesic with a very large spectrum of possible applications.
Saliva
Saliva composition and functions: a comprehensive review, 2008
Pain and Opioids
The International Association for the Study of Pain's uses this definition to describe pain:
“Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”
Pain is usually transitory, lasting only until the noxious stimulus is removed or the damage or pathology has healed, but some painful conditions may persist for years. Traditionally, we can distinguish acute and chronic pain looking at the interval of time from onset:
- Acute pain: self-limiting, usually concordant with the degree of on-going tissue damage, it resolves quickly, with resolution of the injury.
- Chronic pain:not self-limiting, it lasts a long time.
The most efficient drugs to alleviate severe pain are opioid receptor agonists, such as morphine or its surrogates: psychoactive chemical substances.
Opioids have long been used to treat acute pain (such as post-operative pain).They have also been found to be employed in palliative care to alleviate the disabling, chronic, severe pain of terminal conditions.
The opioid drugs, in addition to cause profound analgesia, have the potential to produce mood changes, physical dependence, tolerance and a hedonic ('rewarding') effect which may lead to compulsive drug use.
Opioids produce effects on neurons by acting on receptors located on neuronal cell membranes.
There are three major types of opioid receptor: μ ( mu ) δ ( delta ) and κ ( kappa ). These receptors belong to the large family of receptors which possess 7 transmembrane-spanning domains, and they are coupled with several types of G-proteins that produce inhibitory effects in neurons.
Opioids have actions at two sites, the presynaptic nerve terminal and the postsynaptic neuron.
- The postsynaptic actions of opioids are usually inhibitory.
- The presynaptic action of opioids is to inhibit neurotransmitter release, and this is considered to be their major effect in the nervous system.
Neurotransmitter release from neurons is normally preceded by depolarisation of the nerve terminal and Ca++ entry through voltage-sensitive Ca++ channels. Drugs may inhibit neurotransmitter release by a direct effect on Ca++ channels to reduce Ca++ entry, or indirectly by increasing the outward K+ current, thus shortening repolarisation time and the duration of the action potential. Opioids produce both of these effects because opioid receptors are apparently coupled via G-proteins directly to K+ channels and voltage-sensitive Ca++ channels. Opioids also interact with other intracellular effector mechanisms, the most important being the adenylate cyclase system .

Pain is normally associated with increased activity in primary sensory neurons induced by strong mechanical or thermal stimuli, or by chemicals released by tissue damage or inflammation. Primary sensory neurons involved in pain sensation release predominantly substance P and glutamate in the dorsal horn of the spinal cord. Nociceptive information is transmitted to the brain via the spinothalamic tracts. This ascending information can activate descending pathways, from the midbrain periaqueductal grey area, which exert an inhibitory control over the dorsal horn.
Opioid receptors are present in both the central and peripheral nervous systems. Within the central nervous system, opioids have effects in many regions that are involved in pain transmission and control, including primary afferent neurons, spinal cord, midbrain and thalamus. In the peripheral nervous system, actions of opioids in both the myenteric plexus and submucous plexus in the wall of the gut are responsible for the powerful constipating effect of opioids. In peripheral tissues such as joints, opioids act to reduce inflammation.

Under pathological conditions, the endogenous opioid system is activated: opioid peptides produce analgesia by actions at several levels of the nervous system, in particular, inhibition of neurotransmitter release from the primary afferent terminals in the spinal cord and activation of descending inhibitory controls in the midbrain.
Opioids – mechanisms of action
Opioid analgesics: comparative features and prescribing guidelines, 1996
Endogenous opioid peptides
There are three well-characterized families of opioid peptides produced by the body: enkephalins, endorphins, and dynorphins.
Enkephalins are the most powerful family, they interact with high affinity with both the mu and delta- opioid receptors.
The are two forms of enkephalin: one containing leucine and the other containing methionine; both are products of the proenkephalin gene:
- Met-enkephalin has Tyr-Gly-Gly-Phe-Met.
- Leu-enkephalin has Tyr-Gly-Gly-Phe-Leu.
Because of their high intrinsic efficacy, enkephalins need to occupy a smaller proportion of opioid receptors than morphine to provoke the same antinociceptive responses.
Central administration of enkephalins appears to activate a strong, but short, analgesic response due to their rapid inactivation by the concomitant action of two membrane-bound zinco-ectopeptidases which are co-located with opioid receptors, namely: neutral ecto-endopeptidase ( hNEP ) and ecto-aminopeptidase-N ( hAP-N ).
Increasing the lifetime of circulating enkephalins, released in response to nociceptive stimuli, by inhibiting their degradation is, therefore, an effective method to increase their bioavailability and so to enhance their physiological actions and particularly their analgesic potency.
This is the method used by another opioid peptide: opiorphin.
Endogenous opioids
Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways, 2006
Structure of Opiorphin
Opiorphin is an endogenous opioid present in human saliva, that originates from the N-terminal region of the protein PROL1 (proline-rich, lacrimal 1).
Its levels in human saliva, samples collected from young healthy individuals, ranged from 2.8 to 25.9 ng/ml.
It is a polypeptide consisting of 5 amino acids: Gln-Arg-Phe-Ser-Arg
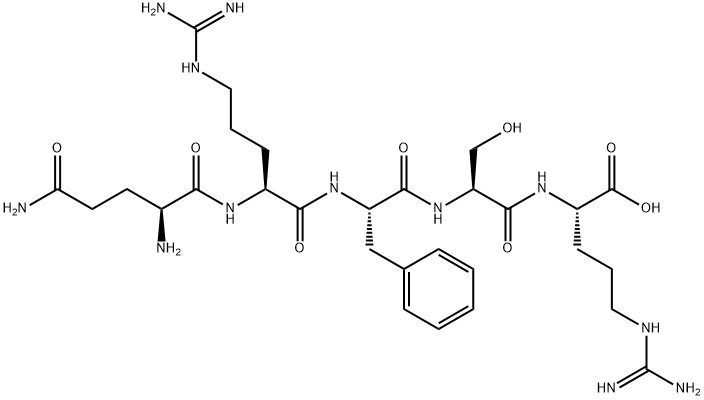
- IUPAC name: (2S,5S,8S,11S,14S)-14,17-diamino-8-benzyl-2,11-bis(3-guanidinopropyl)-5-(hydroxymethyl)-4,7,10,13,17-pentaoxo-3,6,9,12-tetraazaheptadecan-1-oic acid
- Molecular formula: C29H48N12O8
- Molar mass: 692.77 g mol−1
Quantification of opiorphin in human saliva, 2011
Functions
1) Analgesic
Previously we said mammalian zinc ectopeptidases play an important role in turning off neural and hormonal peptide signals at the cell surface, in particular they limit the effects of enkephalins.
In the 2006 study Human Opiorphin, a natural antinociceptive modulator of opioid-dependent pathways is reported the discovery of Opiorphin as a previously uncharacterized physiological inhibitor of enkephalin-inactivating zinc ectopeptidases in humans and it is demonstrated its activity in vitro; this QRFSR peptide inhibits the two enkephalin-catabolizing ectoenzymes: hNEP and hAP-N.
So opiorphin displays potent analgesic activity in chemical and mechanical pain models by activating endogenous opioid-dependent transmission.
Its function is closely related to the rat sialorphin peptide, which is an inhibitor of pain perception and acts by potentiating endogenous μ- and δ-opioid receptor-dependent enkephalinergic pathways.
In the study it was also demonstrated the functional specificity in vivo of human Opiorphin; especially they prove how Opiorphin-derived peptide displays potent analgesic activity in vivo in rat pain model, comparing it to the use of morphine.
But the function of opiorphin is not simply equivalent to other opioids, first of all morphine.
Comparing the occurrence of adverse effects with emphasis on the side-effect profile at equi-analgesic doses of opiorphin and morphin, opiorphin causes minimal adverse morphine-associated effects.
The analgesic response induced by opiorphin preferentially requires activation of endogenous mu-opioid pathways. However, in contrast to exogenous mu-opioid agonists such as morphine, opiorphin, does not develop significant abuse liability or antinociceptive drug tolerance after subchronic treatment.
Systemically active human opiorphin is a potent yet non-addictive analgesic without drug tolerance effects, 2010
2) Antidepressant
In addition to pain control, endogenous opioid pathways are also implicated in the modulation of emotion-related behaviors. Opiorphin in fact is also able to elicit antidepressant-like effects, mediated via delta-opioid receptor-dependent pathways, by modulating the concentrations of endogenous enkephalin released in response to specific physical and/or psychological stimuli.
Enkephalins are associated with the regulation of pain transmission and also of emotional homeostatic equilibrium . So there is a potentiation by opiorphin of the enkephalinergic pathway that might also influence emotional states inducing antidepressive-like behavior.
Human opiorphin is a naturally occurring antidepressant acting selectively on enkephalin-dependent delta-opioid pathways, 2010
3) Role in erectile physiology
As the opiorphins are released into the bloodstream, they also have the potential to act on organs distal to their sites of synthesis.
Opiorphins can relax corporal smooth muscle ( CSM ), and thereby cause an erection (or at higher levels, a priapic-like condition).
This action is mediated through their ability to inhibit endogenous neutral endopeptidase ( NEP ) activity and, therefore, to enhance the activity of peptide agonists bound to the cell membrane, which induce relaxation in CSM cells through activation of G-protein-coupled receptors ( GPCRs ) signaling pathways.

The Role of Opiorphins in Urogenital Smooth Muscle Biology, 2010
Conclusion
Discovery of Opiorphin is extremely exciting for being a natural endogenous peptide, in the context of endogenous opioidergic pathways.
Opiorphin, by inhibiting the destruction of endogenous enkephalins, which are released according to the painful stimulus, activates restricted opioid pathways specifically involved in pain control, contributing to a greater balance between analgesia and side-effects than found with morphine. In fact opiorphin could give rise to new analgesics with potencies similar to morphine but with fewer adverse effects than opioid agonists. Its chemical optimization, to generate functional derivatives with better bioavailability properties than the native peptide could lead to a potent class of physiological type analgesics.
In addition, as the protein shows also antidepressant-like actions, opiorphin or optimized derivatives is a promising single candidate to treat disorders that include both pain and mood disorders, particularly depression.