Lavoro svolto da: KLEVIS KADRIU
TESINA PER MEDICINA DI LABORATORIO: Prof:(Gianpiero PESCARMONA)
Introduction:
Background
Melanoma is a malignancy of pigment-producing cells (melanocytes) located predominantly in the skin, but also found in the eyes, ears, GI tract, leptomeninges, and oral and genital mucous membranes. Melanoma accounts for only 4% of all skin cancers; however, it causes the greatest number of skin cancer–related deaths worldwide. Early detection of thin cutaneous melanoma is the best means of reducing mortality.
Pathophysiology
The sequence of events in which normal melanocytes transform into melanoma cells, referred to as melanomagenesis, is poorly understood. It likely involves a multistep process of progressive genetic mutations that (1) alter cell proliferation, differentiation, and death and (2) impact susceptibility to the carcinogenic effects of ultraviolet radiation.1 Recent data suggest multiple pathways of melanoma pathogenesis, with melanomas in sun-protected skin (trunk) developing in association with a high nevus count and intermittent ultraviolet radiation as opposed to those developing on sun-exposed skin in patients with low nevus counts and chronic sun exposure.

BACKGROUND: Some melanomas form on sun-exposed body sites, whereas others do not. We previously proposed that melanomas at different body sites arise through different pathways that have different associations with melanocytic nevi and solar keratoses. We tested this hypothesis in a case-case comparative study of melanoma patients in Queensland, Australia. METHODS: We randomly selected patients from among three prespecified groups reported to the population-based Queensland Cancer Registry: those with superficial spreading or nodular melanomas of the trunk (n = 154, the reference group), those with such melanomas of the head and neck (n = 77, the main comparison group), and those with lentigo maligna melanoma (LMM) (n = 75, the chronic sun-exposed group). Each participant completed a questionnaire, and a research nurse counted melanocytic nevi and solar keratoses. We calculated exposure odds ratios (ORs) and 95% confidence intervals (CIs) to quantify the association between factors of interest and each melanoma group. RESULTS: Patients with head and neck melanomas, compared with patients with melanomas of the trunk, were statistically significantly less likely to have more than 60 nevi (OR = 0.34, 95% CI = 0.15 to 0.79) but were statistically significantly more likely to have more than 20 solar keratoses (OR = 3.61, 95% CI = 1.42 to 9.17) and also tended to have a past history of excised solar skin lesions (OR = 1.87, 95% CI = 0.89 to 3.92). Patients with LMM were also less likely than patients with truncal melanomas to have more than 60 nevi (OR = 0.32, 95% CI = 0.14 to 0.75) and tended toward more solar keratoses (OR = 2.14, 95% CI = 0.88 to 5.16). CONCLUSIONS: Prevalences of nevi and solar keratoses differ markedly between patients with head and neck melanomas or LMM and patients with melanomas of the trunk. Cutaneous melanomas may arise through two pathways, one associated with melanocyte proliferation and the other with chronic exposure to sunlight.
The RAS/mitogen-activated protein kinase pathway sends external growth-promoting signals to the nucleus. BRAF, a critical serine/threonine kinase in this pathway, is frequently activated by somatic mutation in melanoma. Using a cohort of 115 patients with primary invasive melanomas, we show that BRAF mutations are statistically significantly more common in melanomas occurring on skin subject to intermittent sun exposure than elsewhere (23 of 43 patients; P<.001, two-sided Fisher's exact test). By contrast, BRAF mutations in melanomas on chronically sun-damaged skin (1 of 12 patients) and melanomas on skin relatively or completely unexposed to sun, such as palms, soles, subungual sites (6 of 39 patients), and mucosal membranes (2 of 21 patients) are rare. We found no association of mutation status with clinical outcome or with the presence of an associated melanocytic nevus. The mutated BRAF allele was frequently found at an elevated copy number, implicating BRAF as one of the factors driving selection for the frequent copy number increases of chromosome 7q in melanoma. In summary, the uneven distribution of BRAF mutations strongly suggests distinct genetic pathways leading to melanoma. The high mutation frequency in melanomas arising on intermittently sun-exposed skin suggests a complex causative role of such exposure that mandates further evaluation.
Primary cutaneous melanoma may develop in precursor melanocytic nevi (ie, common, congenital, and atypical/dysplastic types), although more than 60% of cases are believed to arise de novo (ie, not from a preexisting pigmented lesion).
The development of melanoma is multifactorial and appears to be related to multiple risk factors, including fair complexion, excessive childhood sun exposure and blistering childhood sunburns, an increased number of common and dysplastic moles, a family history of melanoma, the presence of a changing mole or evolving lesion on the skin, and, importantly, older age.
Criteria for the early recognition of malignant melanoma include appreciation of variegation in color and irregularity of lesion border and pigment pattern. Application of these criteria should result in the diagnosis of cutaneous melanoma in its premetastatic surgically curable phase.
Cutaneous melanoma is rapidly becoming a potentially curable cancer if it is detected and properly treated in an early phase of development. Unlike other cancers, which are usually hidden from detection until they are relatively large or metastatic disease has occurred, cutaneous melanoma is readily detectable simply by examining the skin. Information is now available that will be useful in selecting individuals at greatest risk. The most important melanoma risk factors (in decreasing order of importance) for a given individual are as follows: a persistently changed or changing mole, adulthood, irregular varieties of pigmented lesions (including dysplastic moles and lentigo maligna), a congenital mole, Caucasian race, a previous cutaneous melanoma, a family history of cutaneous melanoma, immunosuppression, sun sensitivity, and excessive sun exposure. Selective screening and appropriate treatment of individuals who have these risk factors may reduce the morbidity and mortality of cutaneous melanoma.

BRAF_KInase

Despite important advances in the treatment of melanoma, the prognosis for advanced disease remains discouraging. This fact, in combination with a worldwide epidemic of melanoma among persons of white skin type, has focused attention on identifying melanoma in its early, surgically curable stages. Attention has also been directed toward pinpointing which persons are at increased risk for melanoma to reduce risk where possible and to aid early diagnosis. Essentially all epidemiologic studies have identified an increased number of melanocytic nevi as an important risk factor in the development of melanoma, but controversy has arisen concerning the risk associated with certain types of nevi, particularly "dysplastic" nevi. We review melanoma risk factors and examine the relationship between melanocytic nevi and melanoma to clarify for primary care physicians what is "known" (non-controversial) and what is "unknown" (controversial). We propose a working definition of an atypical mole phenotype and outline an approach to managing high-risk patients.
Frequency
United States
The incidence of melanoma has more than tripled in the white population during the last 20 years, and melanoma currently is the sixth most common cancer in the United States. Approximately 62,480 Americans (34,950 men and 27,530 women) will develop invasive cutaneous melanoma in 2008, with an estimated additional 54,020 or more cases of melanoma in situ. The incidence may be higher due to melanoma underreporting to cancer registries, particularly for tumors that are diagnosed and managed in the outpatient setting. The current lifetime risk for developing invasive melanoma is 1 case per 60 Americans, a 2000% increase since 1930. This risk rises to 1 case per 32 Americans if noninvasive melanoma in situ is included.
International
Melanoma incidence has continued to increase worldwide, with the highest incidence in Australia and New Zealand. The most recent analysis of global cancer statistics, from 2002, demonstrated a prevalence of 37.7 cases per 100,000 men and 29.4 cases per 100,000 women in Australia and New Zealand, compared with 6.4 cases per 100,000 men and 11.7 cases per 100,000 women in North America.
Estimates of the worldwide incidence, mortality and prevalence of 26 cancers in the year 2002 are now available in the GLOBOCAN series of the International Agency for Research on Cancer. The results are presented here in summary form, including the geographic variation between 20 large "areas" of the world. Overall, there were 10.9 million new cases, 6.7 million deaths, and 24.6 million persons alive with cancer (within three years of diagnosis). The most commonly diagnosed cancers are lung (1.35 million), breast (1.15 million), and colorectal (1 million); the most common causes of cancer death are lung cancer (1.18 million deaths), stomach cancer (700,000 deaths), and liver cancer (598,000 deaths). The most prevalent cancer in the world is breast cancer (4.4 million survivors up to 5 years following diagnosis). There are striking variations in the risk of different cancers by geographic area. Most of the international variation is due to exposure to known or suspected risk factors related to lifestyle or environment, and provides a clear challenge to prevention.
Mortality/Morbidity
While melanoma accounts for roughly 4% of all skin cancers, it is responsible for more than 74% of skin cancer deaths. In the United States, one person each hour dies from metastatic melanoma. Treatment of melanoma in its early stages provides the best opportunity for cure.
United States: An estimated 8420 deaths will occur in 2007 (5400 men and 3020 women).
Each year, the American Cancer Society estimates the number of new cancer cases and deaths expected in the United States in the current year and compiles the most recent data on cancer incidence, mortality, and survival based on incidence data from the National Cancer Institute, Centers for Disease Control and Prevention, and the North American Association of Central Cancer Registries and mortality data from the National Center for Health Statistics. Incidence and death rates are age-standardized to the 2000 US standard million population. A total of 1,437,180 new cancer cases and 565,650 deaths from cancer are projected to occur in the United States in 2008. Notable trends in cancer incidence and mortality include stabilization of incidence rates for all cancer sites combined in men from 1995 through 2004 and in women from 1999 through 2004 and a continued decrease in the cancer death rate since 1990 in men and since 1991 in women. Overall cancer death rates in 2004 compared with 1990 in men and 1991 in women decreased by 18.4% and 10.5%, respectively, resulting in the avoidance of over a half million deaths from cancer during this time interval. This report also examines cancer incidence, mortality, and survival by site, sex, race/ethnicity, education, geographic area, and calendar year, as well as the proportionate contribution of selected sites to the overall trends. Although much progress has been made in reducing mortality rates, stabilizing incidence rates, and improving survival, cancer still accounts for more deaths than heart disease in persons under age 85 years. Further progress can be accelerated by supporting new discoveries and by applying existing cancer control knowledge across all segments of the population.
Analysis of US Surveillance, Epidemiology, and End Results (SEER) data from 1969-1999 demonstrated a disproportionate burden of melanoma deaths among middle-aged and older white men. While melanoma mortality rates have fallen 39% in women and 29% in men aged 20-44 years over this period, they have increased 66% in men aged 45-64 years and 157% in older men (>65 y).10 Incidence data generally parallel mortality data and have shown a 3-fold increase in middle-aged men and a 5-fold increase in older men over a similar period. Encouragingly, a stable-to-reduced melanoma incidence rate has been noted in younger age groups in the United States, which may be a result of primary prevention campaigns aimed at reducing excessive sun exposure over the past 30 or more years, although the full impact of primary prevention strategies on melanoma incidence and mortality will not be apparent for several decades.
Worldwide: Individuals with cutaneous melanoma have higher survival rates in developed countries (91% in US SEER registries and 81% in Europe) than in developing countries (approximately 40%). Increased educational efforts in developed areas result in earlier diagnosis, treatment, and potential cure of thinner lesions. Worldwide, 160,000 new cases of melanoma were estimated to occur in 2002, with 41,000 deaths reported.9
Race
Melanoma is primarily a malignancy of white individuals. African American persons develop melanoma approximately one twentieth as frequently as white persons, and the prevalence in Hispanic persons is approximately one sixth of that in white persons. However, mortality rates are higher in African Americans and Hispanics, who are more likely to have acral melanoma and advanced disease at presentation.
Sex
In the United States, invasive melanoma has a higher female predilection from birth to age 39 years (1 in 389 women compared with 1 in 656 men). However, from age 40 years and older, melanoma in men predominates, occurring in 1 in 41 men compared with 1 in 61 women over a lifetime.7 Worldwide, of the 160,000 new cases estimated to have occurred in 2002, women were affected slightly more than men (male-to-female ratio, 0.97:1). Conversely, of the estimated 41,000 worldwide deaths in 2002, more occurred in men than in women (male-to-female ratio 1.2:1).
Age
The median age at melanoma diagnosis is 53 years; however, it is the most common cancer in women aged 25-29 years and is second only to breast cancer in women aged 30-34 years. The most striking differences in melanoma incidence and mortality occur in individuals older than 65 years, although modest differences in age-specific incidence and mortality are notable in persons older than 50 years.
Older individuals are both more likely to acquire and to die from melanoma; thus, elderly persons should be a primary target for secondary melanoma prevention, including early detection and screening. Treatment options in elderly persons may also be limited because of comorbid medical conditions, an inability to tolerate adverse medication effects or toxicity, the increased likelihood of drug interactions, and potential exclusion from clinical trials based on age criteria.
Melanoma accounts for the majority of skin cancer deaths worldwide and has dramatically increased in incidence over the past half-century. Despite recent trends showing improved survival, and stabilization of incidence rates in younger Americans, melanoma incidence and mortality continue to rise unabated in older individuals, particularly in men over age 65. Efforts at early clinical detection of melanoma in older individuals should take into account the differences in melanoma subtypes in older individuals, potentially reduced access to medical specialists in this population, as well as comorbidities that may affect ability to undergo treatment for advanced disease. Secondary melanoma prevention should be focused on targeted education to older men and their spouses for early detection and reduction of mortality in this extremely high-risk group.
Clinical
History
A new or changing mole or blemish is the most common warning sign for melanoma. Variation in color and/or an increase in diameter, height, or asymmetry of borders of a pigmented lesion are noted by more than 80% of patients with melanoma at the time of diagnosis. Symptoms such as bleeding, itching, ulceration, and pain in a pigmented lesion are less common but warrant an evaluation. Again, because the majority of cutaneous melanoma arises de novo (ie, not in association with a precursor nevus), the wholesale removal of melanocytic nevi is not warranted for melanoma prevention. However, individuals with numerous moles (common or dysplastic) or a family history of melanoma should be educated regarding the importance of skin self-examination for early detection of skin cancer.http://www.scienzaonline.com/medicina/img/melanoma
Information regarding the changes noted in the ABCDE criteria listed below is relevant to the patient's history. Physician and patient education regarding the warning signs of early melanoma (particularly the superficial spreading subtype) has been achieved successfully through the use of the ABCDE criteria for a changing mole,which are as follows:
Asymmetry: Half the lesion does not match the other half.
Border irregularity: The edges are ragged, notched, or blurred.
Color variegation: Pigmentation is not uniform and may display shades of tan, brown, or black; white, reddish, or blue discoloration is of particular concern.
Diameter: A diameter greater than 6 mm is characteristic, although some melanomas may have smaller diameters; any growth in a nevus warrants an evaluation.
Evolving: Changes in the lesion over time are characteristic; this factor is critical for nodular or amelanotic (nonpigmented) melanoma, which may not exhibit the classic criteria above.
http://www.worldwidewounds.com/2002/march/Naylor/images/Fig-1-Melanoma-Back.jpg


The combination of routine physician examination of the skin coupled with self-examination provides a realistic opportunity for the identification of early malignant melanomas. Removal of such thin lesions can significantly reduce the mortality rate from this potentially serious form of cutaneous cancer.
CONTEXT: The incidence of cutaneous melanoma has increased over the past several decades, making its early diagnosis a continuing public health priority. The ABCD (Asymmetry, Border irregularity, Color variegation, Diameter >6 mm) acronym for the appraisal of cutaneous pigmented lesions was devised in 1985 and has been widely adopted but requires reexamination in light of recent data regarding the existence of small-diameter (< or =6 mm) melanomas. EVIDENCE ACQUISITION: Cochrane Library and PubMed searches for the period 1980-2004 were conducted using search terms ABCD and melanoma and small-diameter melanoma. Bibliographies of retrieved articles were also used to identify additional relevant information. EVIDENCE SYNTHESIS: Available data do not support the utility of lowering the diameter criterion of ABCD from the current greater than 6 mm guideline. However, the data support expansion to ABCDE to emphasize the significance of evolving pigmented lesions in the natural history of melanoma. Physicians and patients with nevi should be attentive to changes (evolving) of size, shape, symptoms (itching, tenderness), surface (especially bleeding), and shades of color. CONCLUSIONS: The ABCD criteria for the gross inspection of pigmented skin lesions and early diagnosis of cutaneous melanoma should be expanded to ABCDE (to include "evolving"). No change to the existing diameter criterion is required at this time.
The ABCDEs have the greatest diagnostic accuracy when used in combination. Lesions exhibiting these features should be considered potential melanoma, although severely atypical nevi may be difficult to distinguish clinically. More recent use of the "ugly duckling" warning sign, wherein skin examination is focused on recognition of a pigmented or clinically amelanotic lesion that simply looks different from the rest, may assist with detection of lesions that lack the classic ABCDE criteria (eg, nodular, amelanotic, or desmoplastic melanomas).
Early detection is crucial to improve melanoma prognosis. Different diagnostic guides such as the ABCD rule (asymmetry [A], irregularity of borders [B], unevenness of distribution of color ©, and diameter [D]) have been proposed to identify melanoma, but their efficacy in real life is questionable. We investigated the recognition process of melanoma by dermatologists to use as a model to improve self-detection in the general population and to train students and general practitioners. OBJECTIVES: To understand the major principles of the recognition process of nevi and melanoma unconsciously used by dermatologists. DESIGN: Prospective survey recording the immediate perceptions of dermatologists of the morphologic features of the lesion and intuitive diagnostic opinion about 4036 consecutive resected nevi and melanoma. SETTING: One hundred thirty-five volunteer dermatologists in their daily practices. MAIN OUTCOME MEASURES: Perceptions of the image best explaining the diagnostic opinion and best predicting the final diagnosis by univariate and multivariate analysis. RESULTS: The immediate diagnostic opinion of the dermatologist is mainly explained by an unconscious reference to the overall pattern compared with the common nevi, but also compared with the other nevi of the individual (the "ugly duckling sign"). The dermatologist's ability to discriminate between nevi and melanoma relies on the assessment of the overall pattern, the ugly duckling sign, and the knowledge of a recent change. A separate or combined analysis of individual morphologic criteria such as ABCD does not seem to play a major role in this recognition process. CONCLUSIONS: Persons most skilled at the clinical detection of melanoma seem to unconsciously rely on cognitive (overall pattern) and comparative (ugly duckling sign) processes rather than an algorithm of morphologic criteria (ABCD). These concepts could be tested in the medical training of general practitioners and education of the general population, where they might be more efficient than algorithms such as the ABCD criteria.
Physical
Four major clinicopathologic (or histogenetic) subtypes of primary cutaneous melanoma have been identified. These include superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. Distinction among the subtypes is based on histologic growth pattern (predominantly junctional in lentiginous types vs buckshot scatter in superficial spreading vs nodular), anatomic site, and degree of sun damage. The pattern of sun exposure varies between the types (sustained in lentigo maligna vs intermittent in superficial spreading). Whether the melanoma subtype affects the overall prognosis remains controversial. However, molecular analysis has demonstrated different patterns of cell death, oncogene expression, gene amplification, and BRAF mutation frequency among the 4 main histogenetic types.
Different patterns of cell proliferation and death and oncogene expression in cutaneous malignant melanoma.
Ninety-six cutaneous melanomas (CMs) were investigated aiming at finding differences, if any, among the main four clinicopathological types, for Bcl-2, c-myc and p53 protein expression, and for tumor cell proliferation and death indices. Proliferation was assessed by calculating the mitotic index (MI, number of mitoses) and the MIB1 labelling index (M-LI, number of MIB1+ nuclei), and tumor cell death by calculating the apoptotic index (AI, number of apoptoses) among 1000 tumor cells. CMs were subdivided into thin (<1 mm) and intermediate thickness (1-4 mm) tumors. Bcl-2 expression did not significantly change among different types. c-myc Expression decreased especially in thicker superficial spreading (SSM) and lentigo maligna melanoma (LMM) types. p53 Expression was higher in nodular melanoma (NM) and in acral lentiginous melanoma(ALM), which also showed the highest degrees of proliferation. AI was significantly higher in thin rather than in intermediate thickness SSMs, LMMs and ALMs (8.4 vs. 2; 6.1 vs. 2.3, and 5.8 vs. 3.6, respectively). AI was low in thin (1.7) and intermediate thickness (1.9) NMs, which also showed high MI (3.9 and 4.5, respectively), and M-LI (16.7 and 2.9, respectively). Thin and intermediate thickness ALMs also showed high MI and M-LI (4.1 vs. 5.2 and 11.3 vs. 14.6, respectively). Bcl-2 is among genes which inhibit apoptotic death, whereas c-myc and p-53 genes promote this process. In CMs, no relation was found between Bcl-2 expression, MI, PI, and AI. All SSMs, LMMs, and ALMs with a high AI showed a high c-myc expression and were negative for p53. c-myc, Although highly expressed, did not promote a significant apoptotic death in NM type. Bc12, c-myc, and p53 were not equally expressed nor equally related to tumor cell turnover in all CMs, suggesting their different influence on the various types and stages, and the role of other factors in CM growth control.
Gene amplifications characterize acral melanoma and permit the detection of occult tumor cells in the surrounding skin.
Acral melanoma (AM) is commonly distinguished from superficial spreading melanoma (SSM), the most common type of melanoma, by its clinical presentation as well as its ethnic distribution. However, justification for such a distinction is controversial because of histological overlap and lack of prognostic significance. We analyzed chromosomal aberrations of 15 AMs and 15 SSMs that were comparable for tumor thickness and patient age, using comparative genomic hybridization. All AMs had at least one (mean, 2.0) gene amplification, significantly more than the SSMs, in which only 2 of 15 (13%) had one amplification each (P < 0.0001). At least 15 different genomic regions were amplified in AM. These involved small portions of chromosomal arms, sometimes including known oncogenes implicated in melanoma. The most frequently amplified regions in AMs occurred at 11q13 (47%), 22q11-13 (40%), and 5p15 (20%). Comparison of the amplification levels of invasive and noninvasive portions of the tumors using fluorescence in situ hybridization suggested that amplifications occurred before the formation of the invasive portion. The finding of amplifications of 11q13 in three of five additional cases of AM in situ further supports the notion that amplifications arise early in the progression of AM. Very significantly, we found isolated melanocytes with amplifications in the epidermis up to 3 mm beyond the histologically recognizable extent of the melanomas in 5 of 15 invasive AMs. In conclusion, our data show that AM is a distinct type of melanoma characterized by focused gene amplifications occurring early in tumorigenesis, and that malignant cells are present beyond the histologically detectable boundary, thereby revealing one mechanism of local recurrence.
BRAF point mutations in primary melanoma show different prevalences by subtype.
To elucidate the biological significance of activating mutations of BRAF in human malignant tumors, we performed a mutation analysis using 43 cell lines established from tumors that had developed in several kinds of human organs. Because the same V599E point mutation was observed in three of six melanoma cell lines and no such mutations were observed in other types of cancers, we focused further on melanoma, performed mutation analyses of NRAS, KRAS, CTNNB1, and p16/p14(ARF) in these cell lines, and found one NRAS mutation and three p16/p14(ARF) mutations. We further searched for mutations of BRAF and NRAS in 35 primary sporadic melanomas from 35 Japanese patients and detected the V599E BRAF point mutation in only nine (26%) of them. Significant differences in mutation frequency were observed among four histological subtypes; four (50%) of eight superficially spreading melanoma and five (33%) of 15 acral lentiginous melanoma had the mutation, whereas none of 12 other types (six nodular melanoma, five lentigo melanoma, and one mucosal melanoma) had it. The BRAF mutation was observed frequently even in small lesions, indicating that activation of this gene may be one of the early events in the pathogenesis of some melanomas.
With the exception of nodular melanoma, the growth patterns of the other subtypes are characterized by a preceding in situ (radial growth) phase that lacks the biologic potential to metastasize and may last from months to years before dermal invasion occurs. While all in situ melanoma may not necessarily progress to invasive melanoma, complete excision is recommended to prevent invasion and effect cure.
Superficial spreading melanoma characteristics are as follows:
It is most common on the trunk in men and women and on the legs in women; this subtype is most commonly seen in individuals aged 30-50 years. See: 
Superficial spreading melanoma manifests as a flat or slightly elevated brown lesion with variegate pigmentation (ie, black, blue, pink, or white discoloration).
It is generally greater than 6 mm in diameter.
Irregular asymmetric borders are characteristic.
Histologically, it is characterized by buckshot (pagetoid) scatter of atypical melanocytes within the epidermis.
Nodular melanoma characteristics are as follows:
This subtype occurs in 15-30% of patients.
It is seen most commonly on the legs and trunk.
Rapid growth occurs over weeks to months; this subtype is responsible for most thick melanomas.
Melanoma and tumor thickness: challenges of early diagnosis.
OBJECTIVE: To test the basic assumption of campaigns for early diagnosis of melanoma, ie, prognosis is correlated with the delay in the diagnosis. DESIGN: Prospective study of the correlation between delays to diagnosis, assessed using a questionnaire, and the Breslow thickness as a prognosis marker. SETTING: Dermatology departments in France. PATIENTS: Five hundred ninety consecutive patients, referred within 12 weeks after resection of cutaneous melanoma. MAIN OUTCOME MEASURES: Assessment of 5 successive time intervals from the first time the patients realized that they had a lesion until the resection of the melanoma, and results of the correlation between each time interval and tumor thickness (Breslow). RESULTS: There is a positive but weak correlation between tumor thickness and the delay to identify a lesion as suspicious (r = 0.17; P = .009). However, this delay tends to be short for the thickest tumors. There is a negative correlation between tumor thickness and the delay to seek medical attention (r = -0.20; P<.001). This delay was shorter for nodular melanoma. No correlation is found between melanoma thickness and physicians' delays. CONCLUSIONS: Poor prognosis can be accounted for by aggressive rapidly growing tumors rather than by delays. In well-informed populations, campaigns for early diagnosis of melanoma may thus no longer have a major impact on prognosis, unless they are focused on subgroups less accessible to information and medical care.
Early detection of thick melanomas in the United States: beware of the nodular subtype.
Incidence and mortality of melanoma in the United States have risen steeply. Part of this mortality increase may be related to late detection of biologically aggressive nodular melanomas. We determined trends in distribution of thin and thick melanoma, with emphasis on the histopathologic subtype nodular melanoma. METHODS: Surveillance, Epidemiology, and End Results melanoma incidence data for whites were obtained for 1988 through 1999 and stratified according to histologic subtype: lentigo maligna melanoma, superficial spreading melanoma, nodular melanoma, other, and not otherwise specified (NOS); thickness: 0-0.99 mm, 1.00 mm-1.99 mm, and > or =2.0 mm; patient age (0-49, 50+); gender; and year (1988-1991, 1992-1995, 1996-1999). Comparison of tumor thickness between strata was defined by year of diagnosis, sex, age, and histologic subtype. RESULTS: The number of new melanoma cases in a 3-year period increased 60% from 1988-1991 (n = 9132) to 1996-1999 (n = 14 575). The proportion of thick melanomas (> or =2 mm) remained relatively stable during the 12 study years. Nodular melanoma comprised 9% of all recorded cases but 34% of melanomas 2 mm or larger, including melanoma not otherwise specified (NOS), and nearly 50% of all melanomas 2 mm or larger when NOS cases were excluded. In contrast, superficial spreading melanoma was almost uniformly diagnosed as an early tumor, mostly (77%) presenting as thin melanoma (<1 mm) and with only 7% presenting as thick melanoma (> or =2 mm). CONCLUSIONS: A substantial number of thick melanomas in the United States are of the nodular subtype, and median thickness of nodular melanoma has not changed during the 12 years of study. New strategies are needed to decrease the incidence of thick melanoma in the United States.
It manifests as a dark brown-to-black papule or dome-shaped nodule, which may ulcerate and bleed with minor trauma; it may be clinically amelanotic (ie, not pigmented).
It tends to lack the typical ABCDE melanoma warning signs and, thus, may elude early detection. More commonly, it exhibits elevation, ulceration with bleeding, or both at presentation.
Histologically, it lacks a radial growth phase.
Lentigo maligna melanoma characteristics are as follows:
The incidence of lentigo maligna subtypes (in situ and invasive) appears to be rising in the United States.
It is typically located on the head, neck, and arms (chronically sun-damaged skin) of fair-skinned older individuals (average age 65 y). See :

It grows slowly over 5-20 years.
The in situ precursor lesion is usually large (>1-3 cm in diameter), present for a minimum of 10-15 years, and demonstrates macular pigmentation ranging from dark brown to black, although hypopigmented (white) areas are common within lentigo maligna. Dermal invasion (progression to lentigo maligna melanoma) is characterized by the development of raised blue-black nodules within the in situ lesion.
Histologically, it is characterized by a predominantly junctional confluent proliferation of melanocytes and extension along adnexal structures. Solar elastosis is typically prominent.
Acral lentiginous melanoma characteristics are as follows:
This is the least common subtype of melanoma (2-8% of melanoma cases in white persons).
It accounts for 29-72% of melanoma cases in dark-skinned individuals (ie, African American, Asian, and Hispanic persons) and, because of delays in diagnosis, may be associated with a worse prognosis.
Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of california cancer registry data, 1988-93.
Cutaneous malignant melanoma occurs less frequently among non-White populations than among Whites. As a result, little is known about the incidence and epidemiology of melanoma among other race/ethnicity groups. Data from the California Cancer Registry (United States) among 879 Hispanic, 126 Asian, and 85 Black men and women diagnosed with melanoma in 1988-93 were analyzed and compared with data for 17,765 non-Hispanic White cases. Average, annual, age-adjusted incidence rates per 100,000 population were 17.2 for men (M) and 11.3 for women (W) for non-Hispanic Whites; 2.8 (M), 3.0 (W) for Hispanics; 0.9 (M), 0.8 (W) for Asians; and 1.0 (M), 0.7 (W) for non-Hispanic Blacks. Among men, melanoma occurred on the lower extremity for 20 percent of Hispanics, 36 percent of Asians, and 50 percent of Blacks compared with nine percent of non-Hispanic Whites, with similar but less pronounced differences in site distribution by race/ethnicity for women. Among men, melanoma was diagnosed after it had metastasized to a remote site for 15 percent of Hispanics, 13 percent of Asians, and 12 percent of Blacks, compared with six percent of non-Hispanic Whites. Among women, seven percent of Hispanics, 21 percent of Asians, and 19 percent of Blacks were diagnosed with late-stage melanoma compared with four percent of non-Hispanic Whites. Although histologic type was not specified for nearly half of the cases, Hispanic, Asian, and Black patients were more likely than non-Hispanic White patients to have been diagnosed with acral lentiginous melanoma. Melanoma among Hispanics, Asians, and Blacks differs in incidence, site distribution, stage at diagnosis, and histologic type from melanoma among non-Hispanic Whites, and identification of risk factors for melanoma in these race/ ethnicity groups would elucidate further the role of sun and other factors in the etiology of melanoma.
http://www.worldwidewounds.com/2002/march/Naylor/images/Fig-1-Melanoma-Back.jpg
Acral lentiginous melanoma occurs on the palms, on the soles, or beneath the nail plate (subungual variant). See: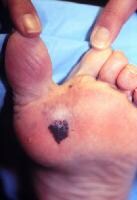
Subungual melanoma may manifest as diffuse nail discoloration or a longitudinal pigmented band within the nail plate.
It must be differentiated from a benign junctional melanocytic nevus of the nail bed, which has a similar appearance.
Pigment spread to the proximal or lateral nail folds is termed the Hutchinson sign, which is a hallmark for acral lentiginous melanoma.
Rare melanoma variants (<5% of melanomas) include (1) desmoplastic/neurotropic melanoma, (2) mucosal (lentiginous) melanoma,(3) malignant blue nevus, (4) melanoma arising in a giant congenital nevus, and (5) melanoma of soft parts (clear cell sarcoma).
Amelanotic melanoma (<5% of melanomas) characteristics are as follows:
This type is nonpigmented and, clinically, appears pink or flesh-colored, often mimicking basal cell or squamous cell carcinoma or a ruptured hair follicle.
It occurs most commonly in the setting of the nodular melanoma subtype or melanoma metastasis to the skin, presumably because of the inability of these poorly differentiated cancer cells to synthesize melanin pigment.
Melanoma can occur on any skin or mucosal surface, although a history of cutaneous melanoma does not appear to increase the risk of developing primary intraocular or mucosal melanoma. Melanoma occurs most commonly on the trunk in white males and the lower legs and back in white females. In African American, Hispanic, and Asian persons, the most common site is the plantar foot, followed by subungual, palmar, and mucosal sites.
Desmoplastic melanoma typically occurs in conjunction with (or in sites typical for) lentiginous types of melanoma (lentigo maligna and acral lentiginous melanoma).
Causes
Superficial spreading melanoma tends to occur at sites of intermittent, intense sun exposure (ie, on trunk in males and legs and back in females). Lentigo maligna melanoma is more prevalent on the chronically sun-damaged skin of the head, neck, and arms. The disease shows an increased worldwide incidence in fair-complexioned individuals living in sunny climates and nearer the equator, suggesting a causative role for ultraviolet radiation.
Primary risk factors for or clinical warning signs of melanoma include the following:
Changing mole (most important clinical warning sign)
Clinical atypical/dysplastic nevi (particularly >5-10)
Large numbers of common nevi (>100)
Large (giant) congenital nevi (>20 cm diameter in an adult)
Previous melanoma
Sun sensitivity/history of excessive sun exposure
Melanoma in first-degree relative(s)
Prior nonmelanoma skin cancer (basal cell and squamous cell carcinoma)25
Male sex
Age older than 50 years
Presence of xeroderma pigmentosum or familial atypical mole melanoma syndrome: These 2 genodermatoses confer a 500- to 1000-fold greater relative risk of developing melanoma.
A fair-skin phenotype (blue/green eyes, blond or red hair, light complexion, sun sensitivity) and the occurrence of blistering sunburn(s) in childhood and adolescence are universal risk factors for melanoma. Individuals with these traits have been the focus of preventive efforts worldwide.
Pregnancy or hormonal therapy with oral contraceptives or hormone replacement does not appear to be a risk factor for melanoma developmen.
Differential Diagnoses:
Laboratory Studies
The most important aspects of the initial workup for patients with cutaneous melanoma are a careful history, review of systems, and physical examination.
Sentinel lymph node biopsy (SLNB) is generally indicated for pathologic staging of the regional nodal basin(s) for primary tumors greater than or equal to 1 mm depth and when certain high-risk histologic features (eg, ulceration, extensive regression, high mitotic rate, angiolymphatic invasion) are present in thinner melanomas.
Published data have shown that baseline and surveillance laboratory studies (eg, lactate dehydrogenase [LDH] level, liver function tests), chest radiography (CXR), and other imaging studies (eg, CT scanning, positron emission tomography [PET] scanning, bone scanning, MRI) are not typically beneficial for stage I/II (cutaneous) melanoma patients without signs or symptoms of metastasis.
A metastatic workup should be initiated if physical findings or symptoms suggest disease recurrence or if the patient has documented nodal metastasis based on results from the SLNB.
Practice guidelines developed by the National Comprehensive Cancer Network support the concept that most melanoma recurrences are diagnosed clinically. The current guidelines state that no further workup (ie, baseline laboratory tests and imaging studies) is required in stage 0 (melanoma in situ) and for asymptomatic patients with stage IA, IB, or IIA melanoma. Chest radiography is considered optional for stage IB-II melanoma, although routine baseline imaging is not recommended for asymptomatic patients with stage IA-IIA melanoma. Further imaging (CT scanning, PET, MRI) should be obtained as clinically indicated (to evaluate specific signs or symptoms) in stage IIB and IIC patients with higher-risk melanoma.
The key components to melanoma follow-up are careful physical examination (with attention to nodes and skin) and review of systems. Patients should be educated in the performance of monthly skin self-examination for early detection of new primary melanoma as well as self lymph node examinations (in those with invasive melanoma). No surveillance studies are recommended for asymptomatic patients with stage IA melanoma, and routine imaging is also not recommended for stages IB and IIA melanoma. Surveillance CXR, LDH, and CBC studies for asymptomatic patients with stage Ib-IV melanoma may be performed on an "optional" basis every 6-12 months at the discretion of the clinician. Advanced imaging studies should be obtained as clinically indicated for confirmation of suspected metastasis or to delineate the extent of disease. Current recommendations do not indicate that baseline or surveillance studies are necessary in patients with melanoma in situ (stage 0) or stage IA disease ( 1 mm thickness).
While abnormal laboratory test results are rarely the sole indicator of metastatic disease, serum LDH levels have been incorporated into the American Joint Committee on Cancer (AJCC) 2002 melanoma staging guidelines for the classification of stage IV (distant) disease. Elevated LDH levels are associated with worse survival in this subgroup. Serum S-100 protein levels may also be useful as a tumor marker in patients with metastatic disease, but this practice is not widely used in the United States.
Imaging Studies
Studies have confirmed that extensive radiologic studies such as CT scanning, MRI, PET scanning, ultrasonography, and bone scanning have an extremely low yield in asymptomatic patients with primary cutaneous melanoma (AJCC stages I and II) and are generally not indicated. However, maintaining a low threshold for obtaining symptom-directed tests is important.
Baseline metastatic staging for melanoma patients with primary tumors greater than 1 mm in depth may include CXR, which typically is repeated every 6-12 months for routine surveillance (optional in the absence of signs or symptoms of metastatic disease).
Procedures
The criterion standard for melanoma diagnosis is histopathologic examination of clinically suggestive skin or mucosal lesions. An excisional biopsy (or deep saucerization technique) with narrow margins is preferred when possible. In the case of lentigo maligna, a broad, paper-thin shave biopsy or multiple smaller biopsies may be the best techniques. The biopsy report should generally include the following:
Tumor thickness (Breslow depth)
Presence of ulceration
Anatomic level of invasion (Clark level)
Presence of mitoses
Presence of regression (associated with lower rates of sentinel node positivity and improved disease-free survival)
Lymphatic/vessel (lymphovascular) invasion or vascular involvement
Host response (tumor-infiltrating lymphocytes)
Immunohistochemical staining for lineage (S-100, homatropine methylbromide 45 [HMB-45], melan-A/Mart-1) or proliferation markers (proliferating cell nuclear antigen, Ki67) may be helpful in some cases for histologic differentiation from melanoma simulators. Additionally, evidence of lack of maturation with HMB-45 staining and patchy, rather than diffuse, staining with S-100A6 may be helpful for distinguishing spitzoid melanoma from Spitz nevus.
Generally, when an excisional biopsy is performed, 1-3 mm of normal skin surrounding the pigmented lesion should be removed to provide accurate diagnosis and histologic microstaging. Wider margins (>1 cm) could theoretically disrupt afferent cutaneous lymphatic flow and affect the ability to identify the sentinel node(s) accurately in patients eligible for this staging procedure. Some data, however, suggest that accurate mapping is possible after wider excision.
Superficial shave biopsies of suggestive pigmented lesions are discouraged because partial removal of the primary melanoma may not provide an accurate measurement of tumor thickness, which is the most important histologic prognostic factor for cutaneous melanoma. As noted above, an important exception to this rule is the lentigo maligna subtype of melanoma in situ. In the case of lentigo maligna, the risk of misdiagnosis is high if small (partial), deep biopsy specimens are taken. The best diagnostic biopsy technique in this case is often a broad shave biopsy that extends into at least the papillary dermis, which provides the opportunity to exclude microinvasive melanoma and allows for optimal histopathologic interpretation of the tumor.
Histologic Findings
Superficial spreading melanoma has an in situ (radial growth) phase characterized by increased numbers of intraepithelial melanocytes, which (1) are large and atypical, (2) are arranged haphazardly at the dermoepidermal junction, (3) show upward (pagetoid) migration, and (4) lack the biologic potential to metastasize. Lentigo maligna melanoma and acral lentiginous melanoma demonstrate predominant in situ growth at the dermoepidermal junction and with little tendency for the pagetoid scatter of cells.
Dermal invasion confers metastatic potential, although the greatest risk occurs in the setting of a vertical growth (tumorigenic) phase.Tumorigenicity is characterized by a distinct population of melanoma cells with evidence of proliferation (mitoses, MIB-1 staining) and nuclear pleomorphism within the dermis and, possibly, the subcutaneous fat. Lateral intraepidermal extension of melanoma cells occurs in all subtypes except nodular melanoma. Failure of melanocyte maturation and dispersion as the tumor extends downward into the dermis is characteristic of melanoma. Some investigators have defined a vertical growth phase as (1) any dermal nest larger than the largest junctional nest or (2) invasion into either the reticular dermis or band of solar elastosis.
Tumor thickness, as defined by the Breslow depth, is the most important histologic determinant of prognosis and is measured vertically in millimeters from the top of the granular layer (or base of superficial ulceration) to the deepest point of tumor involvement. Increased tumor thickness confers a higher metastatic potential and a poorer prognosis.Analysis of worldwide data has shown that the presence of ulceration microscopically, defined as the loss of epidermis overlying the melanoma, is the next most important histologic determinant of patient prognosis and, when present, should be used to up-stage patients with melanoma.The Clark level is a measurement of tumor invasion anatomically and appears to affect prognosis only in thinner (<1 mm depth) melanomas.
Staging
The melanoma staging system initially developed in 1983 by the AJCC and the International Union Against Cancer (UICC) divided melanoma into 4 stages and incorporated tumor thickness and anatomic level of invasion for stages I and II (localized cutaneous disease), with the later recommendation to follow Breslow depth over Clark level when any discordance arose. Stage III disease involved the regional lymph nodes; stage IV disease included distant skin, subcutaneous, nodal, visceral, skeletal, or CNS metastasis.
Major revisions in the 2002 AJCC/UICC melanoma staging system were made based on a critical analysis of prior versions of the staging protocol. The AJCC formed an international multidisciplinary Melanoma Staging Committee and established a new clinicopathologic database of more than 17,000 patients worldwide to test the validity of the proposed staging changes. Several important modifications in the 2002 AJCC staging system include the incorporation of histologic ulceration and number of lymph nodes involved (instead of size) to better stratify metastatic risk and patient prognosis. In the revised staging system, the Clark level is included only in thin primary tumors (<1 mm depth, stages IA and IB) because its prognostic value is minimal in thicker primary melanoma. Microscopic regional lymph node metastasis as detected by SLNB is differentiated from macroscopic nodal metastasis.
Overall survival (OS) in the staging Table below is based on worldwide AJCC data. The next iteration of the AJCC melanoma staging system is anticipated in 2009.
Treatment:
Medical Care
Numerous adjuvant therapies have been investigated for the treatment of localized cutaneous melanoma following complete surgical removal. No survival benefit has been demonstrated for adjuvant chemotherapy, nonspecific (passive) immunotherapy, radiation therapy, retinoid therapy, vitamin therapy, or biologic therapy. Adjuvant interferon (IFN) alfa-2b is the only adjuvant therapy approved by the US Food and Drug Administration for high-risk melanoma (currently defined as stages IIB, IIC, and III), which is associated with a 40-80% chance of relapse and death. Various experimental melanoma vaccines also show promise in the adjuvant setting.
Interferon alfa trials
In the United States, 3 prospective, multicenter, randomized, controlled trials have been conducted to assess the effect of adjuvant high-dose IFN alfa-2b on relapse-free survival (RFS) and OS rates in patients with high-risk melanoma (primary tumors >4 mm depth and regional nodal disease). The Eastern Cooperative Oncology Group (ECOG) trial 1684 showed an 11% increase (26% to 37%) in RFS rates at 5 years in the IFN-alfa treatment group compared with the observation arm. Similarly, this trial showed an increase in 5-year OS rates from 37% to 46% (median OS 2.78 to 3.82 y) in the treatment arm compared with observation.
The confirmatory Intergroup trial (ECOG 1690) again showed an increase in the estimated 5-year RFS rates from 35% in the observation arm to 44% in the high-dose IFN-alfa arm. No significant benefit in the RFS rate was associated with low-dose IFN. Importantly, no difference in the OS rate was seen in the IFN-treated groups (high- or low-dose) compared with the observation arm. Despite further data analysis that suggested postrelapse salvage therapy with an IFN-alfa–containing regimen may have confounded the OS results (ie, "crossover effect"), the ECOG 1690 trial is largely viewed as a negative study for high-dose IFN effects on OS.
The most recent Intergroup trial (ECOG 1694) compared the use of standard high-dose IFN alfa with GM2 ganglioside vaccine (GMK). The study was closed prematurely due to a significant benefit observed for IFN alfa over GMK for both RFS and OS rates. Hazard ratio analysis revealed that the likelihood of disease relapse and death in patients treated with high-dose IFN was reduced by one third compared with GMK.
A pooled analysis of the 3 ECOG/Intergroup trials (with median follow-up ranging from 2.1-12.6 y) revealed that RFS, but not OS, was significantly prolonged for patients treated with high-dose IFN versus observation. The authors concluded there is "strong evidence for improved RFS and evidence for moderate improvement in OS based on two prospective randomized studies (E1684 and E1694), but not in the pooled analysis" and called for further analysis of predictors of both response and relapse to improve the therapeutic value of high-dose IFN therapy.
In any case, the potential benefits of high-dose IFN must be weighed against its substantial tolerability and toxicity issues, including the yearlong duration of therapy, commonly associated flulike symptoms, and potential for significant adverse reactions.
Data from 2006 suggest that high-dose IFN-induced autoimmunity, as manifested clinically by new-onset vitiligo, and/or serologically by the development of autoantibodies (antithyroid, antinuclear, and anticardiolipin), is associated with prolonged RFS and OS in melanoma patients. The apparent prognostic significance of autoimmunity during high-dose IFN treatment warrants further study.
Melanoma vaccines:
Melanoma vaccines are a theoretically attractive alternative to chemotherapy or immunotherapy with systemic cytokines because they are typically associated with relatively little toxicity (eg, fatigue, myalgias, local inflammatory skin reactions). Melanoma vaccines are a type of specific active immunotherapy based on melanoma cell expression of certain HLA- and tumor-associated antigens. Numerous melanoma-associated antigens have been identified, and which of these are the most important in eliciting the necessary cytotoxic and humoral responses to kill melanoma cells remains unclear. In addition, HLA haplotype restriction (mainly to the A2 allele) limits the use of peptide vaccines in many patients. Most current trials for melanoma vaccines are for advanced disease (stages III and IV); trials aimed at prevention are not yet available.
Vaccine types include whole cell preparations, cell lysates, gangliosides, peptides/proteins, dendritic cell vaccines, and DNA vaccines. Melanoma vaccines may be (1) autologous (killed cell and recombinant types), allogeneic, shed from tumor, defined antigen-directed, or genetically engineered and (2) either polyvalent or univalent in nature. Enhanced delivery systems, such as dendritic cell preparations, DNA-plasmid vectors, and intranodal infusion, are under active study to enhance immunogenicity and host response. Biologic response modifiers such as granulocyte macrophage colony-stimulating factor, interleukin (IL)–2, IL-12, and IFN gamma are often integrated into vaccine strategies. As yet, no large, phase 3 randomized trial has demonstrated a survival advantage for vaccine-treated melanoma patients; however, multiple studies are in progress.
Surgical Care:
Surgery is the primary mode of therapy for localized cutaneous melanoma.
Surgical margins for primary melanoma
The narrowest efficacious margins for cutaneous melanoma have yet to be determined. Surgical margins of 5 mm are currently recommended for melanoma in situ, and margins of 1 cm are recommended for melanomas up to 1 mm in depth (low-risk primaries).In some settings, tissue sparing may be critical and Mohs margin-controlled excision may be appropriate.


Randomized prospective studies show that 2-cm margins are appropriate for tumors of intermediate thickness (1-4 mm Breslow depth), although 1-cm margins have been proven effective for tumors of 1- to 2-mm thickness.Margins of 2 cm are recommended for cutaneous melanomas greater than 4 mm in thickness (high-risk primaries) to prevent potential local recurrence in or around the scar site.
A 2004 prospective study of melanoma greater than or equal to 2 mm thickness (median depth 3 mm) from the United Kingdom suggests that narrower margins (1 cm) result in higher locoregional recurrence compared with wider margins (3 cm), although no difference was noted in melanoma-specific survival between the 2 groups.However, this study has been criticized for combining satellite, in-transit, and regional nodal recurrences as the primary end point and by excluding SLNB (which would have demonstrated existing occult regional nodal metastasis at the time of wide local excision). Likewise, because a 2-cm margin is as efficacious as a 4-cm margin for melanomas of 1-4 mm depth, a 3-cm margin is unlikely to prove more beneficial than a 2-cm margin.
A well-conducted retrospective study of high-risk primary melanomas (>4 mm thickness, median depth 6 mm) showed that excisional margins greater than 2 cm have no effect on local recurrence, disease-free relapse, or OS rates; therefore, a 2-cm margin is likely appropriate in this subgroup.
Mohs micrographic surgery has also been proposed for cutaneous melanoma and has the advantage of providing visualization of 100% of peripheral and deep margins microscopically. While studies have shown no increased local recurrence for Mohs surgery compared with historical controls, much of the data stem from thinner tumors with a lower risk of local recurrence and metastasis. Mohs surgery may have certain "niche" indications, including melanomas located the head, neck, hands, or feet. Mohs surgery may prove useful in completely removing subclinical tumor extension in certain subtypes of melanoma in situ, such as lentigo maligna and acral lentiginous melanoma in situ.
Elective lymph node dissection
Prophylactic lymph node dissection for primary cutaneous melanoma of intermediate thickness initially was believed to confer a survival advantage on patients with tumors of 1-4 mm in depth. Subsequently, prospective randomized clinical trials have shown no survival benefit for elective lymphadenectomy for melanomas of varying thicknesses on the extremities and marginal, if any, benefit for nonextremity melanomas.
The 10-year follow-up data from 2 of the trials conducted by the World Health Organization and the Melanoma Intergroup now suggest a survival benefit for certain subsets of patients studied. In particular, patients in the World Health Organization trial who had occult metastasis detected at the time of wide local excision and immediate elective node dissection had a significantly better 5-year survival rate (48%) compared with those who underwent delayed (therapeutic) lymph node dissection when lymphadenopathy became apparent clinically (27%).However, the differences in OS rates for all patients who had delayed lymph node dissection were not statistically significant compared with the immediate node dissection group.
SLNB/dissection
Lymphatic mapping and sentinel node biopsy have effectively solved the dilemma of whether to perform regional lymphadenectomy (in the absence of clinically palpable nodes) in patients with thicker melanomas (1 mm in depth) and in those of less than 1 mm depth with adverse features (eg, ulceration, lymphovascular invasion, mitotic rate 1 mm2).
SLNB for cutaneous melanoma was developed in the early 1990s to allow a selective approach to identifying individuals with occult regional nodal metastasis through localization of the first-draining, or sentinel, node. The success of the technique is based on the concept that cutaneous lymphatic flow is well-delineated in melanoma and that the histology of the sentinel node is characteristic of the entire lymph node basin (ie, a negative sentinel node obviates the need for further lymph node dissection). Both of these concepts were borne out in initial and subsequent studies of the staging technique.
Preoperative radiographic mapping (lymphoscintigraphy) and vital blue dye injection around the primary melanoma or biopsy scar (at the time of wide local excision/reexcision) is performed to identify and remove the initial draining regional node(s).
The sentinel node is examined for the presence of micrometastasis using both routine histology and immunohistochemistry; if present, a therapeutic or completion lymph node dissection (CLND) is performed. A negative sentinel node biopsy result prevents the morbidity associated with an unnecessary lymphadenectomy.
Sentinel node status (positive or negative) is the most important prognostic factor for recurrence and is the most powerful predictor of survival in melanoma patients. In a study of 612 patients with cutaneous melanoma (stage I/II), negative results from SLNB were associated with a nearly 60% increase in 3-year disease-free survival compared with positive SLNB results.Current AJCC 2002 melanoma staging and National Comprehensive Cancer Network clinical practice guidelines advocate pathologic staging of the regional lymph nodes for cutaneous melanoma of greater than 1 mm depth along with microstaging of the primary melanoma as the most complete means of staging.
While SLNB certainly enhances metastatic staging for patients with intermediate-thickness and deeper primary melanomas and provides a more accurate determination of the patient's prognosis, its therapeutic role has yet to be established.The results of the Multicenter Selective Lymphadenectomy Trial (MSLT), the Florida Melanoma Trial, and the Sunbelt Melanoma Trial should help to determine whether SLNB provides a therapeutic benefit in patients with cutaneous melanoma.
The third of 5 planned analyses of the MSLT-1 has been published.This interim analysis of the subset of 1269 patients with intermediate-thickness melanoma (1.2-3.5 mm) demonstrated no overall (melanoma-specific) survival differences in the group that underwent SLNB at the time of primary excision of the melanoma versus the group that underwent wide local excision alone. However, immediate lymphadenectomy in the setting of a positive sentinel lymph node was associated with improved 5-year survival compared with delayed CLND in patients who developed macroscopic nodal metastasis following primary excision alone (72% vs 52%, respectively). The risk of death was reduced by one half (hazard ratio, 0.51; 95% confidence interval, 0.32-0.81; P = .004) in the node-positive subset of patients who underwent immediate versus delayed CLND for regional nodal metastasis. OS rates did not differ.
Longer follow-up with continued analysis of this MSLT-1 and other important SLNB trials will help to elucidate the potential therapeutic benefit of early removal of micrometastasis in the regional nodal basin.
Consultations
Surgical oncologist
For sentinel node biopsy, typically performed at the time of wide local excision and following preoperative lymphoscintigraphy
For surgical treatment of regional lymph node disease and soft tissue and/or in-transit recurrence (stage III disease)
For palliative surgical treatment of visceral and CNS metastasis
Medical oncologist
To discuss adjuvant therapy with IFN alfa, experimental melanoma vaccines, or other clinical trials: Patients should be referred to a medical oncologist or melanoma specialist soon after the melanoma diagnosis and treatment in order to optimize the chances for appropriate adjuvant therapy or clinical trial entry.
To discuss and initiate treatment of metastatic melanoma (stage IV) with chemotherapy, high-dose IL-2, concurrent biochemotherapy, or clinical trials, as indicated clinically
Nuclear medicine specialist
For preoperative lymphoscintigraphy if SLNB is performed
For PET scan interpretation
Pathologist/dermatopathologist
For accurate histologic microstaging of primary melanoma
For evaluation of nodal tissue from SLNB for micrometastasis
For confirmation of the diagnosis of disseminated disease
Radiation oncologist
For consideration of local adjuvant treatment of resected regional nodal metastasis with extracapsular extension or resected intransit metastasis
For palliative treatment of distant metastatic disease, particularly bony metastasis or brain involvement (whole brain radiotherapy or stereotactic radiosurgery)
Neurosurgeon - For evaluation for resectable brain metastasis
Medication
High-dose IFN alfa-2b is the only adjuvant therapy approved by the US Food and Drug Administration for high-risk resected melanoma, defined as deep primaries greater than 4 mm in Breslow depth (AJCC stage IIB) and regional lymph node metastasis (stage III). Various trials of low-dose IFN have shown no benefit in disease-free relapse or OS rates. Similarly, multiple melanoma vaccine trials are in progress, predominantly for stage III and IV disease, but they have not demonstrated an OS advantage to date.
Immunomodulatory agents
Enhance host immunity for cancer surveillance and eradication.
Interferon alfa-2b (Intron A)
Protein product manufactured by recombinant DNA technology. Produced naturally by cells in the body to combat infections and tumors. Mechanism of antitumor activity is not clearly understood; however, direct antiproliferative effects against malignant cells and modulation of host immune response may play important roles.
Generally initiated within 56 d of surgery and typically administered by medical oncologists.
Follow-up:
h1.Further Outpatient Care
Patients should be monitored regularly after a diagnosis of cutaneous melanoma, particularly in the setting of thicker tumors, because most metastases occur in the first 1-3 years after treatment of the primary tumor. Annual skin examinations are recommended for life because an estimated 4-8% of patients with a history of melanoma develop new primary melanoma, generally within the first 3-5 years following diagnosis.The risk of new primary melanoma increases in the setting of multiple clinical atypical/dysplastic nevi, family history of melanoma, and atypical mole syndrome or familial atypical mole-melanoma syndrome. Additionally, individual patient risk factors should be taken into account in the determining the frequency of dermatologic surveillance.
The diagnosis of recurrent/metastatic disease and new primary melanoma depends on a routine evaluation schedule that varies according to the following:
Tumor depth
The presence of histologic ulceration
Lymph node status
Results of the examination of the melanoma scar
Results of the examination of regional and distant lymph node basins
The presence of hepatosplenomegaly upon abdominal examination
Mole pattern and examination findings from the entire cutaneous surface for new primaries
Complications
Metastasis may occur locally (within or around the primary site), in the regional lymph node basins, or distally in the following sites:
Remote skin (away from the melanoma scar)
Remote lymph node(s)
Viscera
Skeletal
CNS sites
Disease relapse is seen most commonly in the skin, subcutaneous tissue, and lymph nodes.
Prognosis
Prognosis is multifactorial and primarily depends on (1) tumor thickness, (2) the presence or absence of histologic ulceration, and (3) lymph node involvement (most important).
Cutaneous melanoma (stages I and II)
Thin primaries ( 1 mm) are associated with a 5-year survival rate of 91-95%, depending on the presence or absence of histologic ulceration and a Clark level of greater than III.
Intermediate-thickness melanoma (1.01-4 mm) is associated with a 5-year survival rate of 63-89%, depending on ulceration and the thickness (1.01-2 mm, 2.01-4 mm) of the primary tumor.
Patients with high-risk tumors (>4 mm) have a 5-year survival rate of 67% without ulceration, compared with 45% with an ulcerated primary.
Ulceration significantly reduces survival at each tumor stage, even when regional lymph nodes are involved.
Stage III disease
Regional lymph node metastasis is associated with a 5-year survival rate of 13-69%, depending on the number of nodes involved, microscopic or macroscopic (matted nodes/gross extracapsular extension) disease, and ulceration of the primary melanoma. In-transit metastasis/satellite lesions are associated with a 30-50% 5-year survival rate, with a significantly worse prognosis in the setting of concomitant regional nodal metastasis (10-30%).
A pooled analysis of high-dose adjuvant IFN-alfa trial results from the United States has shown significantly improved disease-free survival for stage III disease; modest improvement in OS has been observed in 2 prospective randomized studies. Melanoma vaccines/biologic response modifiers show promise in prolonging disease-free survival and OS rates in melanoma patients.
Stage IV disease
Prognosis for distant metastatic disease is extremely poor, with median survival of only 6-9 months and 5-year survival rates of 7-19%, depending on the site(s) of metastasis. In general, patients with soft tissue, nodal, and isolated lung metastases have slightly better prognoses than those with other visceral metastases and/or elevated LDH levels. However, survival beyond 1 year occurs in only a minority of stage IV patients.
Systemic chemotherapy is the mainstay of treatment, despite low response rates (<20%), which also tend to be of short duration.
Biochemotherapy, using standard chemotherapeutic agents with biologic response modifiers such as IL-2, IFN alfa, or granulocyte macrophage colony-stimulating factor, has shown limited success in the management of unresectable stage IV melanoma and is under further investigation. High-dose IL-2 alone, or combined with histamine dihydrochloride, has also shown promise in patients with advanced disease.
As with regional nodal disease, numerous trials are investigating the use of melanoma vaccines (with or without biologic response modifiers) in the treatment of disseminated disease. The hope is that data from the many phase 3 trials in progress worldwide will show improvement in survival for patients with advanced melanoma.
Despite advances in the treatment of metastatic disease, detection and treatment of cutaneous melanoma in its thin, early phase remains the best chance for cure.
Patient Education
Educate patients with a history of melanoma regarding the following:
Sun-protective measures (including sun-protective clothing and sunscreens)
Skin self-examinations for new primary melanoma
Possible recurrence within the melanoma scar
Screening of first-degree relatives, particularly if they have a history of atypical moles
Medicolegal Pitfalls
Clinical or histopathologic misdiagnosis of melanoma or a delay in the clinical diagnosis and skin biopsy may result in thicker tumors with an increased risk of metastasis.
Consensus indicates that skin biopsy results from pigmented lesions suggestive of melanoma should be assessed by a pathologist experienced in the interpretation of melanocytic lesions and a dermatopathologist, whenever possible.
Meccanismo patologico del melanoma:
A Novel BH3 Mimetic Reveals a Mitogen-Activated Protein Kinase Dependent Mechanism of Melanoma Cell Death Controlled by p53 and Reactive Oxygen Species.The RAS/BRAF/MEK/ERK mitogen-activated protein kinase (MAPK) pathway is emerging as a key modulator of melanoma initiation and progression. However, a variety of clinical studies indicate that inhibiting the MAPK pathway is insufficient per se to effectively kill melanoma cells. Here, we report on a genetic and pharmacologic approach to identify survival factors responsible for the resistance of melanoma cells to MEK/ERK antagonists. In addition, we describe a new tumor cell selective means to bypass this resistance in vitro and in vivo. By generating a panel of isogenic cell lines with specific defects in the apoptotic machinery, we found that the ability of melanoma cells to survive in the absence of functional MEK relies on an ERK-independent expression of the antiapoptotic factor Mcl-1 (and to a lesser extent, Bcl-xL and Bcl-2). Using computer-based modeling, we developed a novel Bcl-2 homology domain 3 (BH3) mimetic. This compound, named TW-37, is the first rationally designed small molecule with high affinity for Mcl-1, Bcl-xL, and Bcl-2. Mechanistic analyses of the mode of action of TW-37 showed a synergistic tumor cell killing in the presence of MEK inhibitors. Importantly, TW-37 unveiled an unexpected role of the MAPK pathway in the control of reactive oxygen species (ROS). This function was critical to prevent the activation of proapoptotic functions of p53 in melanoma cells, but surprisingly, it was dispensable for normal melanocytes.

Trattamento cancerogeno:
Systemic Radiation Therapy:Similar to internal radiation therapy, systemic radiation therapy uses radioactive isotopes of iodine and strontium [5]. However, in systemic radiation therapy, these substances are not placed in a container but are instead taken orally or injected in the blood stream such that the entire body is exposed to the radioactive substance [5]. This treatment may require a short hospital stay since the patients sweat, saliva, and urine may contain traces of the radioactive material.

Chemotherapy:
1-Bleomycin is also an intercalating chemothearpy agent and can also act as a DNA cleaving agent. Another intercalating agent is Actinomycin D, which is produced by Streptomyces bacterium.

2-Topoisomerase II Inhibitors:The topoisomerase II enzyme contains DNA lyase and DNA ligase function and is involved in relieving DNA supercoiling. More simply, these enzymes cleave, reorient, and religate genomic DNA to relax negative supercoils. This becomes especially useful during replication, when helicase unwinds double stranded DNA, creating supercoils in the region upstream to the replication fork. Without a way to remove these supercoils, replication would cease due to this tangling of DNA. Therefore, topoisomerase II activity is critical for cell proliferation and survival [19]. Topoisomerase II, unlike topoisomerase I (which makes only a single stranded cut), repairs negative supercoils in DNA by catalyzing double stranded breaks in the supercioled twist of DNA (Figure 4). Topoisomerase II then proceeds to remove the supercoils by passing the double stranded DNA through the cleaved gap and then resealing the gap in an ATP mediated process (Figure 4). If the activity of Topoisomerase II is inhibited or altered, cells would be unable to replicate and divide, which makes this enzyme one of the most popular targets of anti-cancer drugs used today [19].
 3-Catalytic Inhibitors:Conversely, catalytic Topoisomerase II inhibitors completely restrict activity of the enzyme upon binding, leading to the inability of topoisomerase II to cleave, reorient, or religate the supercoiled DNA [30]. These drugs bind to the N-terminal region of the ATPase domain, blocking hydrolysis of ATP. Since opening and closing of the clamp (Figure 4), responsible for the enzyme activity, is linked to ATP hydrolysis, binding of drugs to this region inhibits the catalytic turnover of the enzyme [20]. Irinotecan and topotecan both function as catalytic inhibitors.
3-Catalytic Inhibitors:Conversely, catalytic Topoisomerase II inhibitors completely restrict activity of the enzyme upon binding, leading to the inability of topoisomerase II to cleave, reorient, or religate the supercoiled DNA [30]. These drugs bind to the N-terminal region of the ATPase domain, blocking hydrolysis of ATP. Since opening and closing of the clamp (Figure 4), responsible for the enzyme activity, is linked to ATP hydrolysis, binding of drugs to this region inhibits the catalytic turnover of the enzyme [20]. Irinotecan and topotecan both function as catalytic inhibitors.

 4-Anti-metabolites:Anti-nucleotides,a)These types of anti-metabolites mimic the natural nucleotide pyrimidine or purine substrates. For example, 5-fluorouracil is converted to two metabolites in the body 5,5-fluro-deoxyuridine-5-O-monophosphate and 5-fluorooxyuridine monophosphate [31]. The first metabolite, 5,5-fluro-deoxyuridine-5-O-monophosphate is a pyrimidine analogue that acts to inhibit thymidylate synthase, blocking the synthesis of thymine [31]. 5-fluorooxyuridine monophosphate can be incorporated into RNA during cellular RNA synthesis, which results in cell death [31]. Other pyrimidine based drugs include floxuridine, and cytarabine. Some of the purine analogues used include azathioprine, mercaptopurine, thioguanine, fludarabine, and pentostatin.
4-Anti-metabolites:Anti-nucleotides,a)These types of anti-metabolites mimic the natural nucleotide pyrimidine or purine substrates. For example, 5-fluorouracil is converted to two metabolites in the body 5,5-fluro-deoxyuridine-5-O-monophosphate and 5-fluorooxyuridine monophosphate [31]. The first metabolite, 5,5-fluro-deoxyuridine-5-O-monophosphate is a pyrimidine analogue that acts to inhibit thymidylate synthase, blocking the synthesis of thymine [31]. 5-fluorooxyuridine monophosphate can be incorporated into RNA during cellular RNA synthesis, which results in cell death [31]. Other pyrimidine based drugs include floxuridine, and cytarabine. Some of the purine analogues used include azathioprine, mercaptopurine, thioguanine, fludarabine, and pentostatin.
 b)Anti-folates:These drugs inhibit the function of folic acid within the cell. Folic acid is involved in pyrimidine and purine base synthesis. An inhibition of the action of folic acid would hinder replication and DNA repair. Therefore, anti-folates are a particularly valuable chemotherapy drug [32].
b)Anti-folates:These drugs inhibit the function of folic acid within the cell. Folic acid is involved in pyrimidine and purine base synthesis. An inhibition of the action of folic acid would hinder replication and DNA repair. Therefore, anti-folates are a particularly valuable chemotherapy drug [32].
Anti-folates interfere with the production of tetrahydrofolate from folic acid, which in turn inhibits the fabrication of novel nucleotides. This process most effectively damages DNA synthesis since a completely new genomic copy is needed for cell division. RNA transcription and subsequent protein translation are less affected by the deficiency of novel nucleotides available since mRNA can be recycled and used again.
Methotrexate acts as a folic acid analogue, which is based on their structural similarities. It binds and inhibit the enzyme dihydrofolate reductase, preventing the formation of tetrahydrofolate. Tetrahydrofolate is essential for purine and pyrimidine synthesis, so inhibition of its production from folic acid is detrimental not just to DNA and RNA synthesis, but to protein synthesis as well since tetrahydrofolate is also involved in the synthesis of amino acids serine and methionine [32]. (For more information on Methotrexate see Crohn's Disease Chemotherapy)
 5-Radioimmunotherapy:Monoclonal antibodies can also be used to target particular substances to cancerous cells [42]. This antibody binds to a particular cancerous antigen while also conjugated to radioactive isotope to concentrate the radiation to the tumorous site, while decreasing the exposure of normal cells to radiation. Consequently, cancerous cells are killed by the combined action of the radiation and the monoclonal antibody mediated processes [42]. In 2002, Y-90 ibritumomab tiuxetan was the first radioimmunotherapy agent used. It consisted of a monoclonal antibody similar to rituximab which was bound to tiuxetan, which chelates the radionucleotide Y-90 that emits beta radiations [42].
5-Radioimmunotherapy:Monoclonal antibodies can also be used to target particular substances to cancerous cells [42]. This antibody binds to a particular cancerous antigen while also conjugated to radioactive isotope to concentrate the radiation to the tumorous site, while decreasing the exposure of normal cells to radiation. Consequently, cancerous cells are killed by the combined action of the radiation and the monoclonal antibody mediated processes [42]. In 2002, Y-90 ibritumomab tiuxetan was the first radioimmunotherapy agent used. It consisted of a monoclonal antibody similar to rituximab which was bound to tiuxetan, which chelates the radionucleotide Y-90 that emits beta radiations [42].
 6-Mek/Ras Pathway Inhibitors:Another well known signal transduction pathway involved in cell proliferation, cell-cycle arrest, differentiation, and apoptosis is the Ras/Raf/MEK/ERK signalling cascade. Its faulty activation has been characterized to be a common cause to the formation of cancer. These pathways are activated by binding to various growth factors, such as the basic fibroblast growth factor. Like any signal transduction cascade, there are many targets that can be use for targeting in drug therapy [63]. A previously mentioned molecule, sorafenib, has been shown to have a dual function by also reversibly bind to the active site of the raf kinase, inhibiting the phosphorylation cascade characteristic of this signalling pathway [54, 63]. Consequently, sorafenib can be considered as a multi-target drugs (Multi-Target Drugs), since it also acts as an anti-angiogenic agent [54].
6-Mek/Ras Pathway Inhibitors:Another well known signal transduction pathway involved in cell proliferation, cell-cycle arrest, differentiation, and apoptosis is the Ras/Raf/MEK/ERK signalling cascade. Its faulty activation has been characterized to be a common cause to the formation of cancer. These pathways are activated by binding to various growth factors, such as the basic fibroblast growth factor. Like any signal transduction cascade, there are many targets that can be use for targeting in drug therapy [63]. A previously mentioned molecule, sorafenib, has been shown to have a dual function by also reversibly bind to the active site of the raf kinase, inhibiting the phosphorylation cascade characteristic of this signalling pathway [54, 63]. Consequently, sorafenib can be considered as a multi-target drugs (Multi-Target Drugs), since it also acts as an anti-angiogenic agent [54].
