DEFINITION
The retinoid X receptor is a type of nuclear receptor that is activated by 9-cis retinoic acid.
There are three retinoic X receptors (RXR): RXR-alpha, RXR-beta, and RXR-gamma, encoded by the RXRA, RXRB, RXRG genes, respectively.
RXR heterodimerizes with subfamily 1 nuclear receptors including CAR, FXR, LXR, PPAR, PXR, RAR, TR, and VDR.
As with other type II nuclear receptors, the RXR heterodimer in the absence of ligand is bound to hormone response elements complexed with corepressor protein. Binding of agonist ligands to RXR results in dissociation of corepressor and recruitment of coactivator protein, which, in turn, promotes transcription of the downstream target gene into mRNA and eventually protein.

Retinoid X receptors in macrophage biology, 2013 PDF
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage (Width 700 px)


SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation

CELLULAR FUNCTIONS
cellular localization,
biological function
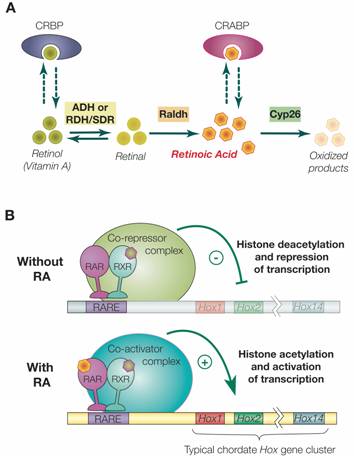
Retinoic acid signaling and the evolution of chordates, 2006
p=. 

RXR heterodimers are either activatory or inhibitory of protein transcription
| RXR | TR | RAR | ER | AR | VDR |
| alpha | + | * | * | + | * |
| beta | + | + | - | * | * |
| gamma | + | * | * | + | * |
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE
RXR-alpha
1. FUNCTION: Nuclear hormone receptor. Involved in the retinoic acid response pathway. Binds 9-cis retinoic acid (9C-RA). ARF6 acts as a key regulator of the tissue-specific adipocyte P2 (aP2) enhancer (By similarity).
2. SUBUNIT: Homodimer or forms a heterodimer with peroxisome proliferator activated receptor gamma called adipocyte-specific transcription factor ARF6. Interacts with NCOA3 and NCOA6 coactivators, leading to a strong increase of transcription of target genes (By similarity). Interacts with SFPQ. Interacts with HCV core protein. Interacts with PELP1. Interacts with SENP6.
3. INTERACTION: O60869:EDF1; NbExp=1; IntAct=EBI-78598, EBI-781301; P55345:HRMT1L1; NbExp=1; IntAct=EBI-78598, EBI-78458; Q9JLI4:Ncoa6 (xeno); NbExp=1; IntAct=EBI-78598, EBI-286271;
4. SUBCELLULAR LOCATION: Nucleus.
5. TISSUE SPECIFICITY: Highly expressed in liver, also found in lung, kidney and heart.
6. DOMAIN: Composed of three domains: a modulating N-terminal domain, a DNA-binding domain and a C-terminal steroid-binding domain.
7. PTM: Sumoylated on Lys-108; which negatively regulates transcriptional activity. Desumoylated specifically by SENP6.
8. SIMILARITY: Belongs to the nuclear hormone receptor family. NR2 subfamily.
9. SIMILARITY: Contains 1 nuclear receptor DNA-binding domain.
10. WEB RESOURCE: Name=Wikipedia; Note=Retinoid X receptor entry; URL=
RXR-beta
- FUNCTION: Nuclear hormone receptor. Involved in the retinoic acid response pathway. Binds 9-cis retinoic acid (9C-RA).
- SUBCELLULAR LOCATION: Nucleus.
- ALTERNATIVE PRODUCTS: Event=Alternative splicing; Named isoforms=2; Comment=Additional isoforms seem to exist; Name=Long; IsoId=P28702-1; Sequence=Displayed; Name=Short; IsoId=P28702-2; Sequence=Not described;
- TISSUE SPECIFICITY: Expressed in a variety of tumor cell lines.
- DOMAIN: Composed of three domains: a modulating N-terminal domain, a DNA-binding domain and a C-terminal steroid-binding domain.
- SIMILARITY: Belongs to the nuclear hormone receptor family. NR2 subfamily.
- SIMILARITY: Contains 1 nuclear receptor DNA-binding domain
RXR molecular biology
Nuclear import
Nuclear Import of the Retinoid X Receptor, the Vitamin D Receptor, and Their Mutual Heterodimer 2005
RXR import to Mitochondria
RXR shortened form and mitochondrial uptake
Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. 2004

figure from: Endocrine regulation of mitochondrial activity: involvement of truncated RXRalpha and c-Erb Aalpha1 proteins. 2003
Limited Degradation of Retinoid X Receptor by Calpain 1996
Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. 1999
Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. 1999
The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats.
Using helix 3 and helix 12 mutants of VDR and
RXR, we provide functional evidence that liganded VDR allosterically modifies
RXR from an apo(unliganded)- to a holo(liganded)-receptor conformation, in the
absence of RXR ligand (with retinoid X receptor (RXR). The RXR-VDR heterodimer in contrast to other members...VDR-mediated transactivation by liganded RXR-VDR has not been fully characterized...unique facet of the intermolecular RXR-VDR interaction, where RXR actively participates...)
VDR TR3 similarities
Thyroid hormones and muscle differentiation
Decreased Retinoid X Receptor-{alpha} Protein Expression in Basal Cells Occurs in the Early Stage of Human Prostate Cancer Development 2004

Peroxisome Proliferator-Activated Receptor -β/δ, -γ Agonists and ResveratrolModulate Hypoxia Induced Changes in Nuclear Receptor Activators ofMuscle OxidativeMetabolism 2010
Polyclonal anti-RXR{alpha} (D-20) sc-553 ({Delta}N 197), anti-RXRß (C-20), anti-RXR{gamma} (Y-20), and anti-p34 cdc2 kinase (H-297) antibodies and polyclonal anti-phospho p44/p42 MAPK (ERK1/ERK2) antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz Biotechnology, CA) and Cell Signaling Technology (Beverly, MA), respectively.
RXR proliferation
Retinoid RXR
Thyroid hormone resistance and increased metabolic rate in the RXR-gamma-deficient mouse. 2000
- Abstract
Vitamin A and retinoids affect pituitary-thyroid function through suppression of serum thyroid-stimulating hormone (TSH) levels and TSH-beta subunit gene expression. We have previously shown that retinoid X receptor-selective (RXR-selective) ligands can suppress serum TSH levels in vivo and TSH-beta promoter activity in vitro. The RXR-gamma isotype has limited tissue distribution that includes the thyrotrope cells of the anterior pituitary gland. In this study, we have performed a detailed analysis of the pituitary-thyroid function of mice lacking the gene for the RXR-gamma isotype. These mice had significantly higher serum T4 levels and TSH levels than did wild-type (WT) controls. Treatment of RXR-gamma-deficient and WT mice with T3 suppressed serum TSH and T4 levels in both groups, but RXR-gamma-deficient mice were relatively resistant to exogenous T3. RXR-gamma-deficient mice had significantly higher metabolic rates than did WT controls, suggesting that these animals have a pattern of central resistance to thyroid hormone. RXR-gamma, which is also expressed in skeletal muscle and the hypothalamus, may have a direct effect on muscle metabolism, regulation of food intake, or thyrotropin-releasing hormone levels in the hypothalamus. In conclusion, the RXR-gamma isotype appears to contribute to the regulation of serum TSH and T4 levels and to affect peripheral metabolism through regulation of the hypothalamic-pituitary-thyroid axis or through direct effects on skeletal muscle.