DEFINITION
The retinoid X receptor is a type of nuclear receptor that is activated by 9-cis retinoic acid.
There are three retinoic X receptors (RXR): RXR-alpha, RXR-beta, and RXR-gamma, encoded by the RXRA, RXRB, RXRG genes, respectively.
RXR heterodimerizes with subfamily 1 nuclear receptors including CAR, FXR, LXR, PPAR, PXR, RAR, TR, and VDR.
As with other type II nuclear receptors, the RXR heterodimer in the absence of ligand is bound to hormone response elements complexed with corepressor protein. Binding of agonist ligands to RXR results in dissociation of corepressor and recruitment of coactivator protein, which, in turn, promotes transcription of the downstream target gene into mRNA and eventually protein.

THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage (Width 700 px)


SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation

CELLULAR FUNCTIONS
cellular localization,
biological function
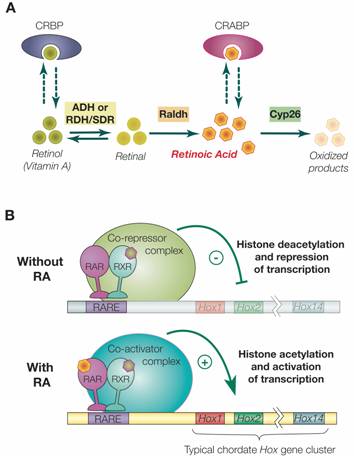
Retinoic acid signaling and the evolution of chordates, 2006


- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE
RXR-alpha
1. FUNCTION: Nuclear hormone receptor. Involved in the retinoic acid response pathway. Binds 9-cis retinoic acid (9C-RA). ARF6 acts as a key regulator of the tissue-specific adipocyte P2 (aP2) enhancer (By similarity).
2. SUBUNIT: Homodimer or forms a heterodimer with peroxisome proliferator activated receptor gamma called adipocyte-specific transcription factor ARF6. Interacts with NCOA3 and NCOA6 coactivators, leading to a strong increase of transcription of target genes (By similarity). Interacts with SFPQ. Interacts with HCV core protein. Interacts with PELP1. Interacts with SENP6.
3. INTERACTION: O60869:EDF1; NbExp=1; IntAct=EBI-78598, EBI-781301; P55345:HRMT1L1; NbExp=1; IntAct=EBI-78598, EBI-78458; Q9JLI4:Ncoa6 (xeno); NbExp=1; IntAct=EBI-78598, EBI-286271;
4. SUBCELLULAR LOCATION: Nucleus.
5. TISSUE SPECIFICITY: Highly expressed in liver, also found in lung, kidney and heart.
6. DOMAIN: Composed of three domains: a modulating N-terminal domain, a DNA-binding domain and a C-terminal steroid-binding domain.
7. PTM: Sumoylated on Lys-108; which negatively regulates transcriptional activity. Desumoylated specifically by SENP6.
8. SIMILARITY: Belongs to the nuclear hormone receptor family. NR2 subfamily.
9. SIMILARITY: Contains 1 nuclear receptor DNA-binding domain.
10. WEB RESOURCE: Name=Wikipedia; Note=Retinoid X receptor entry; URL=
RXR-beta
- FUNCTION: Nuclear hormone receptor. Involved in the retinoic acid response pathway. Binds 9-cis retinoic acid (9C-RA).
- SUBCELLULAR LOCATION: Nucleus.
- ALTERNATIVE PRODUCTS: Event=Alternative splicing; Named isoforms=2; Comment=Additional isoforms seem to exist; Name=Long; IsoId=P28702-1; Sequence=Displayed; Name=Short; IsoId=P28702-2; Sequence=Not described;
- TISSUE SPECIFICITY: Expressed in a variety of tumor cell lines.
- DOMAIN: Composed of three domains: a modulating N-terminal domain, a DNA-binding domain and a C-terminal steroid-binding domain.
- SIMILARITY: Belongs to the nuclear hormone receptor family. NR2 subfamily.
- SIMILARITY: Contains 1 nuclear receptor DNA-binding domain
RXR molecular biology
Nuclear import
Nuclear Import of the Retinoid X Receptor, the Vitamin D Receptor, and Their Mutual Heterodimer 2005
RXR import to Mitochondria
RXR shortened form and mitochondrial uptake
Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. 2004

figure from: Endocrine regulation of mitochondrial activity: involvement of truncated RXRalpha and c-Erb Aalpha1 proteins. 2003
Limited Degradation of Retinoid X Receptor by Calpain 1996
Tumor necrosis factor-alpha-inducible IkappaBalpha proteolysis mediated by cytosolic m-calpain. A mechanism parallel to the ubiquitin-proteasome pathway for nuclear factor-kappab activation. 1999
Oxidative stress decreases G protein-coupled receptor kinase 2 in lymphocytes via a calpain-dependent mechanism. 1999
The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats.
Using helix 3 and helix 12 mutants of VDR and
RXR, we provide functional evidence that liganded VDR allosterically modifies
RXR from an apo(unliganded)- to a holo(liganded)-receptor conformation, in the
absence of RXR ligand (with retinoid X receptor (RXR). The RXR-VDR heterodimer in contrast to other members...VDR-mediated transactivation by liganded RXR-VDR has not been fully characterized...unique facet of the intermolecular RXR-VDR interaction, where RXR actively participates...)
VDR TR3 similarities
Thyroid hormones and muscle differentiation
Decreased Retinoid X Receptor-{alpha} Protein Expression in Basal Cells Occurs in the Early Stage of Human Prostate Cancer Development 2004

Peroxisome Proliferator-Activated Receptor -β/δ, -γ Agonists and ResveratrolModulate Hypoxia Induced Changes in Nuclear Receptor Activators ofMuscle OxidativeMetabolism 2010
Polyclonal anti-RXR{alpha} (D-20) sc-553 ({Delta}N 197), anti-RXRß (C-20), anti-RXR{gamma} (Y-20), and anti-p34 cdc2 kinase (H-297) antibodies and polyclonal anti-phospho p44/p42 MAPK (ERK1/ERK2) antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz Biotechnology, CA) and Cell Signaling Technology (Beverly, MA), respectively.
DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)
External links not available on Wikipedia have to be added here
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage (Width 700 px)


SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
Nuclear import
Retinoid X Receptor Dominates the Nuclear Import and Export of the Unliganded Vitamin D Receptor, 2002
- Liganded and unliganded vitamin D receptors (VDRs) carry out distinct functions; both types of functions require heterodimerization with retinoid X receptors (RXRs). Our recent studies with fluorescent protein chimeras of VDR and RXR, termed GFP-VDR, YFP-RXR, and RXR-BFP, indicated that RXR regulates VDR functions in part by regulating subcellular localization. Here we explored the mechanisms of this regulation. Photobleaching experiments demonstrated that YFP-RXR and both unliganded and liganded GFP-VDR shuttle constantly between nucleus and cytoplasm. To characterize RXR import, we identified a nuclear localization sequence (NLS) in the DNA-binding domain. Mutations in this NLS caused predominant cytoplasmic localization of nlsYFP-RXR and prevented transcriptional activity. The nlsRXR-BFP retained unliganded GFP-VDR in the cytoplasm and reduced baseline transcriptional activity. After calcitriol exposure, however, both GFP-VDR and nlsRXR-BFP entered the nucleus. We characterized receptor export rates and mechanisms using permeabilization experiments. Mutations in the calreticulin binding region slowed both GFP-VDR and YFP-RXR export. Coexpression of RXR-BFP slowed the export of unliganded GFP-VDR, whereas calcitriol treatment tripled the rate of GFP-VDR export. Treatment with leptomycin B, an inhibitor of CRM-1 receptor-mediated export, inhibited export of unliganded GFP-VDR but did not influence export of liganded GFP-VDR or YFP-RXR. Leptomycin B added before calcitriol similarly decreased hormone-induced luciferase activity but was ineffective when added subsequent to calcitriol. These results indicate that the unliganded and liganded VDR interact differently with the import and export receptors and with RXR. Most likely, the regulation of VDR nuclear import by RXR is essential for ligand-independent functions.
biological function
Transcription Factors

Signalling through nuclear receptors, 2002
- homodimers??
- heterodimers
Competition for RXR
As all these factors are active as heterodimers with RXR, RXR availability can became a limiting factor for hormones activity.
RXR scarcity can derive from:
- absolute lack of RXR (diet poor in proteins??)
- relative lack due to sequestration of RXR by an increased number of a specific liganded receptor (TR, VDR; RAR etc)
- Crosstalk between the thyroid hormone and peroxisome proliferator-activated receptors in regulating peroxisome proliferator-responsive genes. 1996
This inhibition was the result of competition between TR alpha and PPAR for limiting amounts of the heterodimerization partner RXR alpha and for binding to the PPRE.
- Crosstalk between the peroxisome proliferator-activated receptor γ (PPARγ) and the vitamin D receptor (VDR) in human breast cancer cells: PPARγ binds to VDR and inhibits 1α,25-dihydroxyvitamin D3 mediated transactivation. 2012
PPARγ was also found to compete with VDR for their binding partner retinoid X receptor alpha (RXRα).
- Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism. II. LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. 2003
Addition of increasing amounts of RXRalpha restored these inhibitory effects in both luciferase and gel shift assays, suggesting the presence of RXRalpha competition.
- Ligand-activated PPARbeta efficiently represses the induction of LXR-dependent promoter activity through competition with RXR. 2006
Collectively, these results suggest that the binding of PPARβ-specific ligand enhances the affinity between RXRα and activated PPARβ and thus may regulate angptl3 gene expression through a DR4 element by competing with LXRα for RXRα.
- Ligand-regulated heterodimerization of peroxisome proliferator-activated receptor α with liver X receptor α. 2015
Complex interactions between the LXRα and PPARα pathways exist, including competition for the same heterodimeric partner, retinoid X receptor α (RXRα).
- Peroxisome Proliferator Activated Receptor-α Association With Silent Information Regulator 1 Suppresses Cardiac Fatty Acid Metabolism in the Failing Heart. 2015
Sirt1 competes with RXRα to dimerize with PPARα, thereby suppressing FA utilization in the failing heart.
- Crosstalk between the thyroid hormone and peroxisome proliferator-activated receptors in regulating peroxisome proliferator-responsive genes. 1996
This inhibition was the result of competition between TR alpha and PPAR for limiting amounts of the heterodimerization partner RXR alpha and for binding to the PPRE.
- Peroxisome proliferator activated receptor-gamma (PPARgamma) represses thyroid hormone signaling in growth plate chondrocytes. 2005
Overexpression of retinoid X receptor-alpha (RXRalpha) partially restored not only the inhibition of transcriptional activation by PPARgamma but also T3-induced hypertrophic differentiation.
- Thyroid hormone receptor does not heterodimerize with the vitamin D receptor but represses vitamin D receptor-mediated transactivation. 1998
No VDR-TR heterodimer binding on any of these VDREs was observed, although, as expected, there was binding by the VDR-RXR complex and strong TR-RXR binding to a consensus thyroid hormone response element.
- Vitamin D interferes with transactivation of the growth hormone gene by thyroid hormone and retinoic acid. 1996
Our data suggest the formation of TR-VDR and RAR-VDR heterodimers with RXR.
- Chromosomal localization of the human retinoid X receptors. 1994
The recently described retinoid X receptors (RXRs) respond to the novel retinoid 9-cis-retinoic acid and also serve as heterodimeric partners for the vitamin D, thyroid hormone, and retinoic acid receptors (VDR, TR, and RAR, respectively).
- Clinical effect of competition
REGULATION
DIAGNOSTIC USE