DEFINITION
The atria and other tissues of mammals contain a family of peptides with natriuretic, diuretic, vasorelaxant, and other properties. The family includes atrial natriuretic peptide (ANP), b-type natriuretic peptide (BNP) and c-type natriuretic peptide (CNP). The peptides share a common 17 amino acid disulfide ring with variable C- and N-terminals. A fourth peptide, urodilatin, has the same structure as ANP with an extension of four amino acids at the N-terminal.
Recently it was discovered also DNP (Dendroaspis natriuretic peptide), and it was isolated from the venom of the Green Mamba snake, Dendroaspis augusticeps. It is not yet classified in the cardiac natriuretic peptide family of mammals even if it has a very similar structure to ANP and BNP and a high affinity for the same receptors.

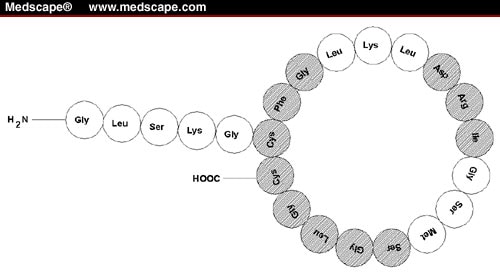
Structures of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). Sequences common to the peptides are indicated in green
GENES
ANP and BNP related genes are on the short arm of chromosome 1 (1p36.21 and 1p36.2) and that of CNP on chromosome 2 (2q24) in humans.
CHROMOSOME 1

CHROMOSOME 2

ATRIAL NATRIURETIC PEPTIDE (ANP)
The circulating (biological active) form of human ANP comprises a 28 amino acid peptide with a 17 amino acid ring closed by a disulfide bond between two cysteine residues. Its amino acid sequence is highly conserved across species.
ANP is present as a single-copy gene and is organized into three exons separated by two introns. The precursor of ANP, preproANP, is converted to proANP, which is a predominant storage form of ANP in specific atrial granules. Next, proANP is cleaved into amino-terminal ANP and biologically active 28-amino acid ANP in mammals. In humans, transmembrane serine protease, corin, has been shown to convert proANP to ANP. Alternative processing of proANP by an unknown protease in the kidney generates a 32-residue peptide called urodilatin, which may be important in regulating renal sodium and water excretion.
In the heart, cardiac myocytes are the predominant cells for ANP production, although ANP may also be synthesized by fibroblasts. Atrium is the most important site for the synthesis of ANP, since the ANP mRNA levels in the atria are 100-fold higher than in the ventricles. Atrial ANP is secreted in a constitutive and regulative manner, whereas ventricular ANP secretion occurs predominantly in a constitutive pathway. Cardiac myocyte stretch resulting from increased intravascular volume is suggested to be a primary stimulus for the release of ANP. Once secreted, ANP perfuses into the coronary sinus, which facilitates distribution to its various target organs in a true endocrine manner. In addition, hormones such as endothelin, angiotensin, and arginine-vasopressin (AVP) stimulate ANP release, as do water immersion and head down tilt.

B-TYPE NATRIURETIC PEPTIDE (BNP)
BNP was initially purified from porcine brain extracts and given the name brain natriuretic peptide.
However, it was subsequently found in much higher concentrations in cardiac ventricles from patients or animals undergoing cardiac stress such as congestive heart failure or myocardial infarction. For this reason, it is currently referred to as BNP or B-type natriuretic peptide, but not brain natriuretic peptide.
The BNP gene is composed of three exons and two introns. In contrast to ANP, BNP has considerable inter-species diversity of amino acid composition. The posttranslational processing of BNP precursors seems to be different from that of ANP, and the processing sites are not conserved between species, resulting in various lengths of BNP.
Human BNP is synthesized as a preprohormone of 134 residues containing a signal sequence that is cleaved to yield a 108-amino-acid prohormone. Additional cleavage by an unknown protease results in an inactive 76-residue amino-terminal fragment and a 32-residue carboxyl-terminal biologically active peptide. Fully processed BNP length varies between species. Human, pig, and dog BNP is 32 amino acids , whereas rat and mouse BNP is 45 amino acids.
BNP is secreted by the ventricles of the heart in response to excessive stretching of heart muscle cells (cardiomyocytes).
The physiologic actions of BNP are similar to ANP and include decrease in systemic vascular resistance and central venous pressure as well as an increase in natriuresis. Thus, the net effect of BNP and ANP is a decrease in blood volume and a decrease in cardiac output.
Although BNP is stored with ANP in atrial granules, BNP is not stored in granules in the ventricles. Instead, ventricular BNP production is transcriptionally regulated by cardiac wall stretch resulting from volume overload. The nuclear transcription factor, GATA 4, plays a dominant role in regulating this process.

C-TYPE NATRIURETIC PEPTIDE (CNP)
CNP is the most highly expressed natriuretic peptide in the brain and is found in high concentrations in chondrocytes and cytokine-exposed endothelial cells. It is not stored in granules. In cultured endothelial cells, its secretion is up-regulated by TNF-α , TGF-β, IL-I, and sheer stress and suppressed by insulin. CNP is the most conserved natriuretic peptide. For instance, both 22- and 53-amino-acid versions of CNP are identical in humans, pigs, and rats. Human proCNP contains 103 residues, and the intracellular endoprotease furin has been shown to process proCNP to the mature 53-amino-acid peptide in vitro. In some tissues, CNP-53 is cleaved to CNP-22 by an unknown extracellular enzyme. Although CNP-22 and CNP-53 elicit similar if not identical functions, their tissue expression differs. CNP-53 is the major form in the brain, endothelial cells, and heart, whereas CNP-22 predominates in human plasma and cerebral spinal fluid.
Unlike ANP and BNP, CNP does not have direct natriuretic activity. This is because CNP is a selective agonist for the B-type natriuretic receptor (NPRB) whereas ANP and BNP are selective for NPRA.
DATABASE
PROTEINS AMINOACIDS PERCENTAGE




RECEPTORS AND MOLECULAR MECHANISM
Guanylyl cyclases exist as soluble and particulate, membrane-associated enzymes which catalyse the conversion of GTP to cGMP, an intracellular signalling molecule. Several membrane forms of the enzyme have been identified up to now; some of them serve as receptors for the natriuretic peptides. These are transmembrane proteins composed of a single transmembrane domain, a variable extracellular natriuretic peptide-binding domain, and a more conserved intracellular kinase homology domain (KHD) and catalytic domain.


Three natriuretic peptide receptors have been identified:
• GC-A or NATRIURETIC PEPTIDE RECEPTOR-A (NPR-A) or NPR-1 is the receptor for ANP and BNP.
The human NPR-A gene is approximately 16 kb, contains 22 exons and 21 introns, and is located on chromosome 1q21–22
It is highly expressed in kidney, adrenal, terminal ileum, adipose, aortic, and lung tissues.
Binding of ANP or BNP to ours receptor causes the conversion of GTP to cGMP and raises intracellular cGMP; as a consequence, cGMP activates a cGMP-dependent kinase (PKG or cGK) that phosphorylates proteins at specific serine and threonine residue which will then induce smooth muscle relaxation.
• C-type natriuretic peptide binds and actives the GC-B or NATRIURETIC PEPTIDE RECEPTOR-B (NPR-B) or NPR-2, and its regulation is similar to that of GC-A.
NPR-B is found in lung, brain, adrenal, kidney, uterus, and ovary tissue.
The human gene spans about 16.5 kb, contains 22 exons, and is located on chromosome 9p21–12.
NPR-A and NPR-B are membrane-bound guanylyl cyclases consisting of an extracellular ligand binding domain, a single hydrophobic transmembrane region, and intracellular kinase homology, dimerization, and carboxyl-terminal guanylyl cyclase domains. The catalytic domain is hypothesized to form a dimer in a head-to-tail arrangement that contains two active sites.
• Another receptor is NPR-C or NPR-3 that functions mainly as a clearance receptor by binding and sequestering ANP from the circulation.
NPR-C is found in atrial, mesentery, placenta, lung, kidney, and venous tissue, in aortic smooth muscle and aortic endothelial cells.
The human gene is located on chromosome 5p14-p13, spans more than 65 kb, and contains eight exons and seven introns.
NPR-C is approximately 30% identical to NPR-A and NPR-B in the extracellular ligand-binding domain but contains only 37 intracellular amino acids.
All natriuretic peptides are bound by this receptor.

PHYSIOLOGICAL FUNCTIONS
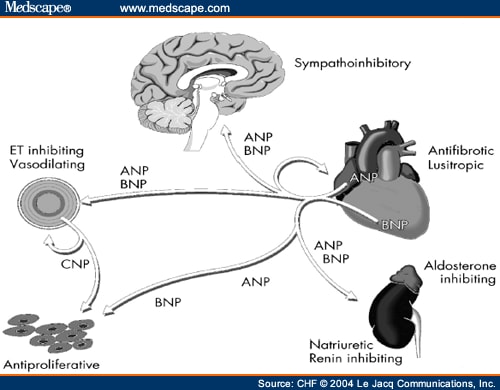
The natriuretic peptides can affect systemic blood pressure by several mechanisms, including modification of renal function and vascular tone, counteracting of the rennin-angiotensina-aldosterone system and action on brain regulatory sites. These systems maintain a balance which ensures relative constancy of body electrolyte and water content and circulatory homeostasis.
Main biologic actions of natriuretic peptides
• Cause of natriuresis and diuresis
• Cause of vasodilation
• Suppression of renin action
• Suppression of aldosterone action
• Suppression of sympathetic activity
• Suppression of antidiuretic hormone
• Inhibition of growth of vascular smooth muscle
THERAPEUTIC AND DIAGNOSTIC APPLICATIONS
Initial studies tested the use of ANP and BNP as potential therapeutic agents for the treatment of congestive heart failure, hypertension, and renal failure. The infusion of synthetic ANP into patients with hypertension or chronic heart failure resulted in elevated sodium and water excretion and decreased blood pressure. Long term (48 h) ANP infusions of patients with acute heart failure resulted in beneficial hemodynamic responses without tolerance, suggesting that ANP injections may be a clinically useful treatment for heart failure. ANP was also used to treat patients with acute renal failure.
Human recombinant BNP mimics the actions of endogenous BNP and has been shown to cause potent vasorelaxation accompanied with increases in natriuresis and diuresis, as well as decreases in plasma aldosterone and endothelin levels in patients with acute heart failure.
The U.S. Food and Drug Administration approved the use of BNP for the treatment of acutely decompensated heart failure in 2001.
The other clinical benefit of natriuretic peptides comes from their diagnostic use. ANP and BNP levels are increased in patients with heart failure and in many patients with hypertension and chronic renal failure because BNP plasma levels correlate more closely than ANP
levels with left ventricular function, a common indicator of heart disease, BNP is considered a better diagnostic marker of heart failure.
Immunoassays that measure the level of BNP or pro-BNP are commonly used clinically to rule out or confirm a heart failure diagnosis in patients. Elevated BNP levels correlate with poor prognoses from other diseases as well (poststroke mortality, postcardiac surgery, atrial fibrillation, as well as the risk of death in patients with heart failure).
The therapeutic uses of CNP have yet to be explored.
Potential use for CNP therapy:
• the treatment of dwarfism, especially acromesomelic dysplasia type Maroteaux (loss of function mutations in NPR-B).
• speed the healing of bone fractures (rats lacking functional PKGII heal much slower than those from wild-type animals.
• CNP may hold promise as a cardiovascular drug because recent evidence indicates that it can prevent cardiac remodeling after myocardial infarction in mice.
Barbara Calvo
Paola Bonaventura