Author : Matteo Maria Ottaviani
INTRODUCTION
Metallothionein (MT) is a family of cysteine-rich, low molecular weight (MW ranging from 500 to 14000 Da) proteins.
There are at least ten known closely related metallothionein proteins expressed in the human body. In humans, large quantities are synthesized primarily in the liver and kidneys, however they have been found at a number of other sites as well. Its production is dependent on availability of the dietary minerals zinc and selenium, and the amino acids histidine and cysteine present in the body.
The four main isoforms expressed in humans are: MT1 (subtypes A, B, E, F, G, H, L, M, X), MT2, MT3, MT4. MT isoforms are classified based on various factors like molecular weight, metal which bind, encoded genes, chromosomes, binding atoms, amino acids environment etc.
Broadly it is classified as major and minor groups.
The major groups are MT-1 and MT-2; these are the unique structure which is identical for the two major isoforms binds 7 atoms of divalent metals like zinc and cadmium.
The MT-3 and MT-4 are minor isoforms which are normally found in specialized cells.
DISTRIBUTION
• MT-I and II are present in all cells throughout the body. They regulate copper and zinc, are involved in cell transcription, detoxify heavy metals, play a role in immune function, and are involved in a variety of G.I. tract functions.
• MT-III is found primarily in the brain and functions as a growth inhibitory factor in the brain. MTIII is located primarily in the central nervous system with small amounts present in the pancreas and intestines. It plays a major role in the development, organization and programmed death of brain cells.
• MT-IV is found in the skin and upper G.I. tract. They help regulate stomach acid pH, taste and texture discrimination of the tongue and help protect against sunburn and other skin traumas.
LOCALIZATION
These cysteine-rich proteins are localized in cytoplasm and some organelles, predominantly in mitochondria, where their presence is sensitively and strictly regulated by the oxidative state induced by mitochondrial respiration.
Lysosomes represent another place of MTs localization. The presence of MT, namely MT-3, is related to lysosomal changes and cell death in neurons under oxidative stress. Relation between iron-catalysed intralysosomal peroxidative reactions, MT protective effect and oxidative stress is suggested in a study by Baird et al. Depending on the cell state, but especially presence of oxidative stress, MTs are rapidly translocated to the nucleus through nuclear pore complexes. MT localized in the nuclei is oxidized there and it is transported to cytosol; this system is balanced. Translocation of MTs to the nucleus is probably connected with protection of the cell against DNA damage and apoptosis and gene transcription during different stages of cell cycle .
The role of nitric oxide in enhancement of MT nuclear localization is connected with scavenging ability of MT with subsequent formation of nitrosothiol, which reduces nuclear as well as cytoplasmic damage by nitric oxide.
MTs have the capacity to bind both physiological (such as zinc,copper, selenium) and xenobiotic (such as cadmium, mercury, silver, arsenic) heavy metals through the thiol group of its cysteine residues, which represents nearly the 30% of its amino acidic residues.
MT was discovered in 1957 by Vallee and Margoshe from purification of a Cd-binding protein from horse (equine) renal cortex. MTs function is not clear, but experimental data suggest MTs may provide protection against metal toxicity, be involved in regulation of physiological metals (Zn and Cu) and provide protection against oxidative stress.

STRUCTURE
Cysteine residues are the most highly conserved followed by the basic amino acids lysine and arginine.

The metal binding domain of MT consists of 20 cysteine residues juxtaposed with Lys and Arg arranged in two thiol-rich globular domains called α and β. Twenty cysteine residues occur in primary sequence in following repetitions: Cys-X-Cys, Cys-Cys-X-Cys-Cys, Cys-X-Cys-Cys, where X is amino acid different from cysteine. Domains are separated by a cysteine-free central part usually called a spacer and composed of a conserved Lys–Lys segment (residues 30 and 31) in the middle of the polypeptide chain.
The α -domain often consists of amino-acids residues No. 32–61, β domain No. 1–31.
N-terminal part is marked as β-domain with three binding sites for divalent ions, usually for Zn(II) or Cd(II), with nine cysteinyl sulphurs.
C-terminal part (α -domain) is capable of binding four divalent metal ions by 11 cysteines with five bridging sulfur atoms.
In summary, the cysteine sulfhydryl groups can bind 7 moles of divalent metal ions per mol of MT, while the molar ratio for monovalent metal ions (Cu and Ag) is twelve.

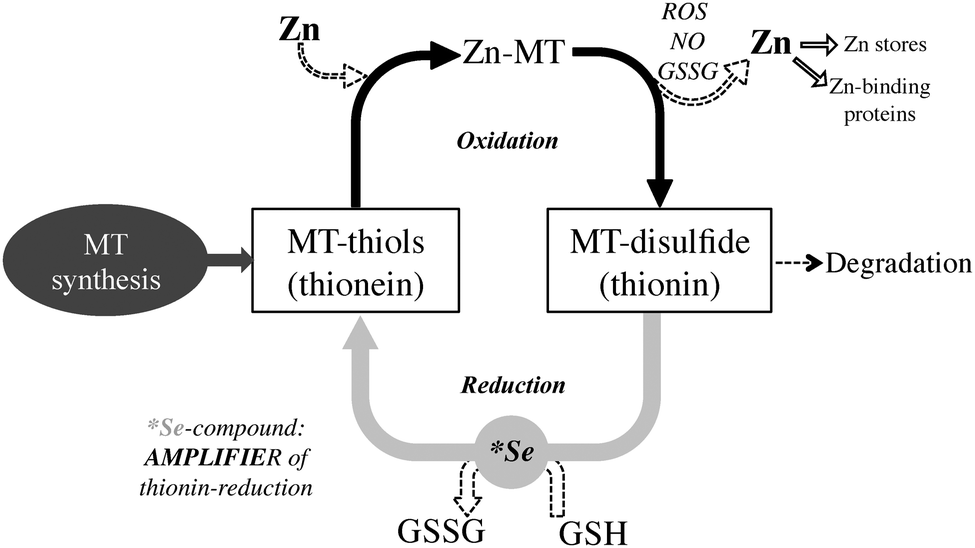
At the center of the mechanism of action of MT is the dynamic interaction of its cysteines with zinc and the redox activity of the sulfur ligand of the cysteines.
The three zinc ions in the N-terminal Zn3S9 cluster and two of the zinc ions in the C-terminal Zn4S11 cluster have two terminal sulfurs and two bridging sulfurs. The remaining two zinc ions in the Zn4S11 cluster have three bridging sulfurs. Thus, one consequence of the cluster structure is the lowering of the affinity for some of the zinc ions. As a result of these properties, metallothionein exists as different species: Zn7T (MT), Zn6T, Zn5T, and Zn4T. The distribution of these species depends on the total concentration of both the protein and zinc ions. Given the low affinity for the seventh zinc ion and the fact that cellular free zinc ion concentrations are only picomolar, Zn7T cannot exist under normal physiological conditions.
Zn6T and Zn5T are the species that participate actively in zinc transfer from or to other proteins.

The effect of binding to metals on the cysteinyl thiols is to reduce the pKa of the cysteines by up to 6 orders of magnitude. As a consequence, the cysteine sulfurs bind to the metals as thiolates. The formation of these metal–thiolate (cysteine) bonds dominates the secondary structure of the protein, so that it may be describe the secondary and tertiary structures present in the native metallated protein as arising primarily as a result of metalinduced folding.


GENE AND EXPRESSION

The genes for MTs are clustered and are located on chromosome 16q12-22 in humans. The MT-2A isoform accounts for 80% of human metallothionein (hMT) expression.
Metallothionein gene expression is induced by a high variety of stimuli, as metal exposure, oxidative stress, glucocorticoids, hydric stress, pH etc. The level of the response to these inducers depends on the MT gene.
MT expression control elements can be functionally subdivided into two categories: basa**l and **inducible. There are several distinct basal sequences, which include the TATA-box, GC-box, and at least two basal level enhancer (BLE) sequences.
MT genes present in their promotors specific sequences for the regulation of the expression, elements as metal response elements (MRE), glucocorticoid response elements (GRE), GC-rich boxes, basal level elements (BLE), and thyroid response elements (TRE).

Gene transcription is initiated when zinc(II) ions associate with metal-regulatory transcription factor-1 (MTF-1).
MTF-1 is a zinc binding protein that contains six Cys2-His2 zinc fingers, the presence of these zinc fingers, along with differences in their respective affinity, make MTF-1 exquisitely sensitive to changes in the concentration of zinc in a cell.
MTF-1 is the only known mediator of the metal responsiveness of MTs. MTF-1 binds to metal-responsive elements (MREs) that regulate MT expression.
MREs are located in the promoter regions of MT genes and are present in multiple copies in the promoter/enhancer regions of almost all metal-inducible MTs.

However, there have been identified other metals, which are able to induce MT transcription and also pose a threat to cells because of their ability to promote carcinogenetic processes, such as Cd2+, Cr6+, and Ni2+. One of their adverse actions is displacing zinc from zinc-saturated MTs or other zinc-binding proteins. Zinc binds to MTF-1 and promotes the formation of a complex containing MTF-1 and p300. The MTF-p300 complex then binds to DNA and triggers a transcription apparatus, leading to MT gene transcription.
MT mRNA is relatively short lived, with maximal levels found hours after zinc is administered. MT mRNA was found primarily in the free polysomal pool, suggesting an intracellular function for the protein. Elements in the untranslated region of the mRNA may direct it to the perinuclear cytoplasm and cytoskeletal-bound polysomes.
Furthermore, in vivo and in vitro evidence has shown that apo MT (thionein) is highly susceptible to proteolysis, whereas zinc and particularly cadmium binding make MT resistant to proteolysis.
The kinetics of induction of MT mRNA by a typical heavy metal ion inducer such as Zn2+ and dexamethasone, a synthetic glucocorticoid, are different. Both inducers lead to rapid induction of MT mRNA, but while the response to metal is biphasic and the level of the mRNA declines after prolonged exposure, the response to the hormone is persistent and once a new elevated steady state level of MT mRNA is achieved, it is maintained as long as the hormone is present heavy metal induction is mediated through the removal of a repressor of MT gene expression.
In the simplest hypothesis the repressor is apothionein, which explains the specificity of the induction response, since only metal ions that bind to the apoprotein can lead to induction. This induction is due to the conversion of apotheionein, the active repressor form, to metallothionein, the inactive repressor form. After a while, the level of MT in the cell is increased, but so does the level of the apoprotein, the putative repressor, leading to a decrease in the transcription rate of the MT genes, and resulting in a transient induction response. Induction by glucocorticoids bypasses this pathway and leads to persistent activation of the genes, as long as, the hormone receptor complex is bound to DNA.
The response of MT expression in animals to the administration of IL-1 and IL-6, interferon γ and other mediators has been documented. This is also true for endotoxin, which acts via initiating cytokines and other mediators. Cytokine-induced metallothionein expression is tissue specific.
It seems that expression of MT is sensitively and strictly regulated by the oxidative state induced in mitochondrial respiration. Deregulation of this state may be connected with many diseases.
ROS increases MT-1 and MT-2 transcriptional responses in a dose-dependent manner, and subsequently mRNA expression and its levels.
Catecholamines are also able to activate MT-1 and MT-2 gene transcription.
MT genes are transcriptionally activated also by redox fluctuations.
MT-1 gene expression is activated as a response to hypoxia.
A novel pathway for MT expression was discovered in the papillary thyroid cancer cell line (KAT5) where MT expression is predated by elevated calcium ions and ERK1/2. Inhibition of calcium as well as ERK1/2 led to blocking of MT expression and progression of G0/G1 to G2/M cell phase.
POLIMORFISMS
MTs are a genetically polymorphous protein family with subfamilies, subgroups, and isoforms.
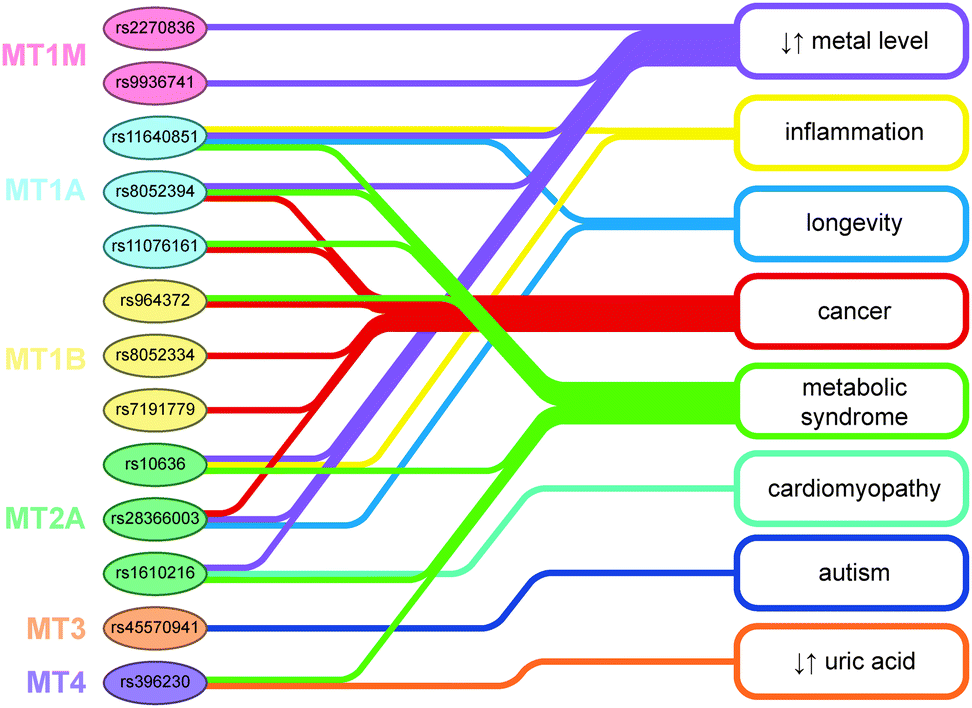
FUNCTIONS
Metal Homeostasis
The β-domain with 9 cysteine residues is capable of binding 3 Cd2+ or Zn2+, or 6 Cu+ and the α-domain with 11 cysteine residues capable of binding 4 Cd2+ or Zn2+, or 6 Cu+.
Support for the role of MT as a metallochaperone is found in the transcription of DNA to RNA, which has been shown to be strongly controlled by exposure to metal ions.
In the case of mammals, of the four isoforms (MT-1 to -4) only two (MT-1 and MT-2) are strongly upregulated by metal ions.
Cell free transcription experiments have shown a number of stresses, including exposure to Cd2+, Cu+ and H2O2 function by displacing naturally bound Zn2+ from MT. These free Zn2+ ions bind to MTF-1 leading to a translocation of MTF-1 from the cytoplasm to the nucleus where the Zn-MTF-1 interacts with MREs leading to upregulation of MT. In this way, MT is capable of countering and deactivating a wide range of insults in order to return an organism to homeostatic balance.
Naturally occurring MTs are usually isolated as either Zn-MT, Cu-MT or as the mixed metal species Zn, Cu-MT and Zn,Cd-MT. In the case of mammals, Zn-MT is the dominant form, however, Cu-MT has been isolated from several sources. Elevated Cu-MT levels have also been found in patients suffering from Wilson’s disease.
At the cellular level both toxic and nontoxic metals are tightly controlled and estimates of free Cu+ and Zn2+ suggest that neither is available in the cytosol. The absence of freely available Cu+ and Zn2+ suggests that an organism is able to exist in this state by using metallochaperones to transport these ions in a controlled manner. MT is considered one such metallochaperone, which is capable of transporting essential Zn2+ and Cu+ to apo-enzymes.
MT is generally understood to coordinate all the group 11 and 12 metals, and is also capable of binding to other metals including: Co2+, Pb2+, Pt2+/4+, Fe2+, As3+, Bi3+ and Tc5+.The binding affinities of MT for metal ions follows closely with the association constant of metal ions for inorganic thiolate ligands (Hg2+ > Cu+ > Cd2+ > Zn2+). Thus MT preferentially coordinates many toxic metals and ultimately this coordination leads to the release of Zn2+, which acts to upregulate the production of MT and returns an organism to homeostatic balance.
MT knockout studies have highlighted the importance of this protein in the homeostasis of Zn2+. For example, MT-null mouse pups fed severely deficient Zn2+ diets, showed delays in kidney development
when compared to wild-type controls. On the other hand, MT-null adult mice challenged with increased Zn2+ showed a greater incidence of pancreatic acinar cell degeneration. These results demonstrate that MT is critical in protecting an organism from the extremes of Zn2+ exposure, acting as both a source and a sink for Zn2+ during deficiency and excess, respectively.
Zinc, in turn, is a key element for the activation and binding of certain transcription factors through its participation in the zinc finger region of the protein. Metallothionein also carries zinc ions (signals) from one part of the cell to another. When zinc enters a cell, it can be picked up by thionein (which thus becomes "metallothionein") and carried to another part of the cell where it is released to another organelle or protein. This system is particularly important in the brain, where zinc signaling is prominent both between and within nerve cells.
Copper toxicity is also of considerable interest, because unlike zinc, free copper is able to catalyze the formation of hydroxyl radicals through Haber-Weiss and Fenton reactions. MT-1 and -2 knockout micE demonstrated that in the absence of inducible-MT, knockout mice were more susceptible to copper Toxicity. Cellular damage is somewhat mitigated by the accumulation of copper in the form of Cu-MT.
MT acts also to buffer the toxic effects of Cd2+ to an organism by sequestering the Cd2+, likely leading to the release of Zn2+, which would act to upregulate MT.
Cadmium (Cd) is an environmental pollutant ranked eighth in the top 20 hazardous substances and the human activity has markedly increased the distribution of Cd in the global environment. Cd is toxic to number of tissues in body. This is also classified as a human carcinogen causing genitourinary disorders like tumors of the lung, prostate, injection site, and other tissues. Most of Cd in the body is bound to a small, cysteine-rich, metal binding protein MT. This protein expression in Cd-induced tumors varies depending on the type and the stage of tumor development. High levels of MT are detected in Cd-induced sarcomas at the injection site and sarcomas metastases are devoid of MT suggest the critical role for protecting human health from Cd toxicity either by neither detoxification nor heavy metal binding.
Control of oxidative stress
The high thiol content of MT makes it an ideal molecule to interact with and inhibit reactive oxygen species (ROS). MT is upregulated by ROS through the antioxidant response element (ARE), a promoter region on the MT gene, ARE-binding transcription factors, as well as MTF-1. As a reducing agent that readily coordinates Zn2+, a proposed function of MT is sensing the presence of incoming oxidants. In this redox cycle, a ROS oxidizes MT leading to Zn2+ release. This Zn2+ is responsible for upregulating Zn-dependent proteins through MTF-1. Either reduction of the previously oxidized MT with glutathione, or complete replacement with de novo MT then leads to a reestablishment of zinc homeostasis. In this manner, MT may either directly interact with the ROS, or in cases where ROS production is the result of a metal ion that readily coordinates MT, such as copper, MT may act to sequester the metal ion and effectively neutralize ROS activity.
In vitro experiments with DNA incubated with MT demonstrated that MT is capable of scavenging free hydroxyl radicals. Scavenging MTs ability is demonstrated for organic radicals.
Connection between hydrogen peroxide and MT level was evidenced. Zn-MT has been shown to be a potent protective and stabilization agent of biomembranes undergoing lipid peroxidation. Released Zn(II) ions themselves can serve as regulators and inhibitors of this process, mainly in the presence of oxidative stress. Zn(II) ions can antagonize the catalytic properties of the redox-active transition metals, such as Cu(I, II) andFe(II, III), which are connected to the formation of superoxide and hydroxyl radicals.
MT thiol clusters are capable of chelating iron (II, III), as has been demonstrated in in vitro experiments with subsequent prevention of Fenton-dependent oxidative reaction catalysis.
MT may serve as a reservoir from which apometalloproteins, including enzymes and zinc finger proteins (transcription factors, signaling and adapter molecules), acquire zinc from MT can be donated to
some transcription factors . The exchange reaction may occur by direct donation of zinc from MT through a protein-protein interaction. Exchange with oxidized glutathione (GSSG) results in monophasic formation of a 1:1 Zn-glutathione (GSH) complex, which may also have a function in zinc mobilization from MT.
GSSG also enhances the transfer rate of zinc from MT to apometalloproteins and increases the number of zinc atoms released. Release of cellular zinc from MT by GSSG would require a low GSH/GSSG ratio, a situation that occurs during oxidative stress. This implies redox control of zinc release from MT.
Cell Proliferation and Tumors
Interactions with transcriptional factors
Although MT is a cytosolic protein in resting cells, it can be translocated transiently to the cell nucleus during cell proliferation and differentiation.
MT regulation during cell cycle progression has been demonstrated in normally cycling cells. Maximal nuclear accretion of MT by two- to three-fold the basal level was found to coincide with the S and G2 phases, while high cytoplasmic expression occurred during late G1 phase and G1/S transition and basal amounts were found in G0 phase.
The perinuclear localization of MT-I mRNA is important for the function of MT in a protective role against DNA damage and apoptosis induced by external stress.
In another study, four-fold increases in MT-II were found in proliferating liver cells compared with those in growth arrest, and there was evidence for translational control as well as a slower rate of MT-II degradation in proliferating cells.
MT induction (by metals, oxidants and electrophiles) could regulate gene expression and cell proliferation by controlling occupancy of zinc binding sites in zinc finger transcription factors. Similarly, other zinc-sensitive processes, such as apoptosis, could be influenced by such cellular events. The transfer of zinc from MT to other zinc metalloproteins is thermodynamically dependent on the Kd involved. Consequently, in some situations, zinc may be transferred from metalloproteins to apothionein. Several in vitro studies have shown that apothionein can remove zinc from zinc finger transcription factors. The result is loss of DNA binding activity, which is regained with free zinc or zinc-containing metallothionnein (Zn-MT).
Hence, Zn-MT may rescue zinc finger proteins from inactivation by other metals, explaining in part its proposed role in metal detoxification.

Zinc is an intracellular mediator of apoptosis, which can interfere with the action of Ca2+. Zinc addition prevents DNA fragmentation and inhibits many proteins connected to apoptosis, such as caspases and calcium-magnesium– dependent proteases. Moreover zinc induces a transcription of the p53 gene, with increased expression of p53 mRNA and protein.
MTs interact with different proteins important for cell cycle regulation. They are the p50 subunits of NF-kB, kinase domain of PKC, and GTPase Rab3A. MT can modulate the biological activity of p53 by zinc(II) exchange. MT-1 and MT-2 regulate the level, activity and cellular location of the transcription factor NF-kB. NF-kB is essential for cell protection from apoptosis induced by TNF and other stimuli. NF-kB activates antiapoptotic genes such as Bcl-2, c-myc, and TRAF-1. Apo-MT-1 (metal-free form of MT-1), but not
MT-1 (MT-1 with metal ion) forms a complex with p53 and, thus increases metal-dependent expression of MREs. Those interactions are important for tumor growth, because activation and/or inactivation of these proteins may mediate the antiapoptotic effect of MTs.

Cancer
The protective role of MT in oxidative stress and metal toxicity suggests that MT may also have a functional role in tumor cell survival and growth. A number of factors are known to be involved in both intrinsic and acquired resistance to antineoplastic agents, and expression of MT in tumor may be one of them.
MT could facilitate tumor cell growth by two potential mechanisms. It can act as a zinc donor to various transcription factors, including tumor suppressor gene products such as p53. In vitro studies have shown that thionein can modulate transcriptional activation of Sp1, a zinc finger transcription factor, thereby suggesting exchanges of zinc between MT and other proteins involved in cell growth. The translocation of MT into the nucleus during the proliferative phase (G1-S) of the cell cycle in human tumors, also support a zinc donor role for MT during tumor growth. Besides, MT is known to inhibit apoptosis, and thus provide resistance to antineoplastic agents. Thus in the second mechanism, MT can protect the cells from radiation and chemotherapeutic agents by virtue of its free radical scavenging property.
A number of studies have shown an increased expression of MT in various human tumors of the breast, colon, kidney, liver, lung, nasopharynx, ovary, prostate, salivary gland, testes, thyroid and urinary bladder. However, MT is down-regulated in certain tumors such as hepatocellular carcinoma and liver adenocarcinoma. Hence, the expression of MT is not universal to all human tumors, but may depend on the differentiation status and proliferative index of tumors, along with other tissue factors and gene mutations
Increased expression of MT has also been observed in less differentiated tumors. Thus, expression of MT may be a potential prognostic marker for certain tumors.
Intra-tumour hypoxia is a feature of various types of cancers, including prostate carcinoma; it is associated with tumour progression, acquisition of anti-apoptic potential and therapeutic resistance.
Proliferation of prostate tumour cells LNCaP and PC-3 was related to the protective effect of MT under hypoxia and its up-regulation is demonstrated. Connection between reactive oxygen species and cell cycle progression has been demonstrated repeatedly. They are produced during cell proliferation. Several biological events can be considered, such as the electron transport in energy metabolism. Endogenous ROS accumulation leads to inhibition of cell proliferation. Antioxidant effects of MT, especially in the nuclei of observed (MT)-null (MT_/_) cells, is obvious. Hypoxia itself is accompanied by oxidative stress and ROS generation. In conclusion, MT regulates cell proliferation through its antioxidant activity and zinc(II) level control.
Chemoresistance of anticancer drugs
Metallothionein as protein with high affinity to metals is capable due to its –SH groups to bind platinum-based cytostatics through platinum and thereby reduce their cytotoxic effect.
Chemoresistance to main platinum antitumor compounds like cisplatin and carboplatin is mediated through two broad mechanisms, first, a failure of a sufficient amount of platinum to reach the target DNA and, second, a failure to achieve cell death after binding of platinum to DNA. Transfer of platinum from cisplatin and carboplatin to MTs results in inactivation of those drugs.
MT cooperates with GSH/GST system; glutathione S-transferases are a family of enzymes catalysing conjugation of anti-tumour drugs such as melphalan, cyclophosphamide and chlorambucil with tripeptide glutathione, the most abundant intracellular thiol.
Metallothionein in Human Disease
MT isomers and Cancer
In Breast Cancer
There are several reports on the expression of certain specific isoforms in various human tumors.
The mRNA of MT-1 series named as A, E, F, G, H, X and MT-3 isoforms but not MT-1B and MT-4 isoforms have been detected in breast cancer tissues.
The MT-2A mRNA transcript which has been reported to be highest among all the functional isoforms detected in breast tissues and is positively correlated with cell proliferation and histological grade.
Expression of MT-1F isoform has also been found to influence histological differentiation in invasive breast cancer since estrogen is known to play important role in breast cancer tumorgenesis, the MT-1E isoform has been postulated to participate in alternative processes that replace the function of estrogen.
It has also been reported that MT-3 isoform overexpression is associated with a poor prognosis for patients with breast cancer.
In Renal Tumor
The renal cell cancer tissue shows three different type of expression as up-regulation of MT-2A, down-regulation of MT-1A and MT-1G transcripts. Expression of the MT-3 isoform has been reported in the tubules of normal kidney and also in renal cell carcinoma along with other isoforms of MT. The expression of the MT-3 isoform in cancerous bladder tissues which was absent in normal bladder tissues, and suggested its use as a potential biomarker for bladder cancer. They have also shown high levels of MT-1X mRNA expression in bladder cancer. The MT-3 isoform which was originally reported as specific to brain has been demonstrated in normal human kidney, renal carcinoma, bladder cancer and prostatic adenocarcinoma.
In Prostate Cancer
In normal prostate tissue, the MT-I A, E, X and MT-2A isoforms were present but there was a down-regulation of the MT-IX isoform in advanced prostate cancer. It was reported that MT-1 and MT-2 isoforms may be related to the proliferative activity of breast, colon and prostate human cancers.
In Papillary Thyroid Cancer
MT isoforms have not been much studied in papillary thyroid cancer. The function of MT1 and MT2 isoforms in papillary thyroid cancer cells (KAT5) demonstrated that KAT5 cells expressed eight functional MT1 and MT2 isoforms induced by cadmium. Elevated calcium and activated ERK1/2 predated MT expression. The alternation in cell cycle disappeared when the expression of MT isoforms was blocked by calcium inhibitor or ERK1/2 inhibitor. Collectively, KAT5 cells express eight functional MT1 and MT2 isoforms in a pathway controlled by calcium and ERK1/2. The elevation of the MT isoforms contributes to the decreased G0/G1 but increased G2-M phase revealed a novel pathway for the expression of the functional MT in papillary thyroid cancer. Bone thyroid cancers are classified as papillary, follicular, medullary, and undifferentiated or anaplastic.
Bone Growth Retardation and MT Isomers
Bone growth retardation, zinc and its binding protein MT are important in regulating growth and development of bone. A study on relationship between dietary Zn and MT interact in regulating bone growth were reported that the MT mice, having lower Zn concentrations in plasma and long bone, showed growth retardation as demonstrated by lower body length gain, shorter and smaller tibia/femur, lower chondrocyte proliferation, reduced metaphysis heights, but increased osteoclast densities on trabecular bone, particularly in mice fed Zn low diet (Zn-L). The mRNA expression of MTI& II was induced in mice fed with the Zn-L diet possibly compensating for Zn limitation that interact between dietary Zn and endogenous MT is important for maximal bone growth, and particularly important in the regulation of Zn pool for bone growth during moderate Zn limitation.
MTs in cancer diagnosis
Associations of MTs with several diseases, including cancer, circulatory and septic shock, coronary artery disease, and Alzheime’s disease, have been found. Further, strong evidence exists that MTs modulate the immune system. A tumor stimulates the remodeling of its microenvironment for its own survival. To protect its own growth and induce angiogenesis, the tumor changes the structure of extracellular matrix and the function of existing cells; it thus chemoattracts immune-system cells, altering their function. MT, because of its antiapoptotic, pro-proliferative, and immunomodulating functions, is discussed as a potential marker of tumor microenvironment remodeling.
MT can serve as a prognostic marker in central nervous system tumors of childhood and adolescence, osteosarcoma, breast cancer, pancreatic islet cell tumors, and tongue squamous-cell carcinoma. MT can also serve as a serum tumor marker in prostate cancer, head and neck tumors, childhood solid tumors, and melanoma. Expression of MT may help to distinguish between benign and malignant tumor, as shown in thyroid tumors, prostatic lesions, GI stromal tumors, and gastric carcinomas.
MTs in cancer therapy
MTs, because of their roles in tumors, can be targeted for cancer therapy. Silencing of MT by short interfering RNA (siRNA) was published by Tarapore et al., who used phage Phi29 motor pRNA as a vehicle to carry siRNA specifically targeted to MT-2A mRNA in ovarian cancers (Tarapore et al., 2011), and by Lai et al., who reported that silencing of the MT-2A gene by siRNA induces entosis (a process involving the invasion of one cell into another, and internalized cells are either degraded by lysosomal enzymes or released) in adherent MCF-7 breast cancer cells (Lai et al., 2010).
Targeting unique mRNA molecules using antisense approaches, based on sequence specificity of double-stranded nucleic acid interactions, should allow for the design of drugs with high specificity for intended targets. Antisense-induced degradation or inhibition of translation of a target mRNA is potentially capable of inhibiting the expression of any target proteins.
Downregulation of MTs by antisense RNA is known to inhibit growth of the tumor cell. This strategy for downregulation of the MT gene in tumors is possible to inhibit their growth and metastasizing in breast cancer cells, leukemia P388 cells, Ehrlich’s carcinoma, sarcoma 180 cells, and nasopharyngeal cancer.
Antisense MT mRNA may induce sensitivity of cancer cells to a heavy-metal–based cytostatic.
Aberrant DNA methylation in histologically healthy mucosae has attracted attention as an indicator of past exposure to carcinogens and as a marker for future risk prediction. Methylation and epigenetics of MT isoforms are also studied with their potential use in cancer therapy. MT-1M was methylated in squamouscell carcinoma, and MT-1E was very commonly methylated in melanoma tissues. Increase in MT gene methylation was observed to correlate with melanoma development, more frequently in primary tumors, and the most frequently in metastases. The resulting silencing might also play a role in the resistance of melanoma to cisplatin, as shown by in vitro experiments.
Other roles of MT in cancer therapy is its protective action during chemotherapy. Cells with developed resistance to heavy-metal–based cytostatics have increased expression of MTs.
Targeting of MTs with antisense RNA for reversal of multidrug resistance was proposed.
In The Central Nervous System
This important protein plays a major role in the defense against neurodegenerative disorders and other injuries, influence tissue architecture and cognition, finally protect against mercury neurotoxicity.
MT has important functions in the central nervous system and brain because MT-1 and MT-2 protect the central nervous system from damage induced by interleukin, 6-aminonicotinamide, kainic acid, and physical injury.
Neuroprotection and Neurodegeneration
Over the last two decades, MT-1/2 and MT-3 have been gaining recognition for their role in neuroprotection and neuroregeneration and therefore are starting to be considered as potential therapeutic agents for several neurological and neurodegenerative disorders.
Zinc binding, ROS scavenging, and promotion of neuronal survival are common features of both proteins with a strong impact in neuroprotection and neuroregeneration.
The zinc binding and antioxidant properties of MT-1/2 and MT-3 are consensual, have been extensively and consistently demonstrated, and explain most of the neuroprotective actions reported for these two proteins. The capacity to promote neuronal survival is consensual for MT-1/2 but has raised controversial results for MT-3, which may be a consequence from the concentrations of the protein used in several different studies. At low concentrations, the protein induces neuronal survival whereas at higher concentrations it has the opposite effect.
Unquestionable anti-inflammatory properties have been attributed to MT-1/2, but there is a lack of reports on anti-inflammatory properties of MT-3.
The recent elucidation of receptor-mediated (LDLR) actions of MT-1/2 makes it difficult to discriminate between receptor mediated actions of MT-1/2 and those arising from its action as a modulator of zinc intracellular levels or as a potent scavenger of ROS. The best demonstrated receptor-mediated actions of MT-1/2 are neurite outgrowth and neuronal survival, but apoptosis and brain inflammation may also be regulated, to some extent, by receptor-mediated actions of MTs. However, the combination between zinc binding and the antioxidant properties of MTs are likely to play an important role on these two features of MT-1/2. In fact, both zinc and ROS are important modulators of apoptosis. Increased levels of free zinc in astrocytes and neurons are considered one of the major causes of death in ischemic and traumatic incidents. Zinc induces cell death in the CNS via apoptotic and necrotic pathways and often encompasses both mechanisms. Elevated zinc concentrations promote the release of proapoptotic proteins such as cytochrome-c and apoptosis-inducing factor from neuronal mitochondria. The neuroinflammatory response characterized by microglia activation and astrogliosis, involving recruitment of leukocytes and production of inflammatory mediators as proinflammatory cytokines and TNF-a has a consequent increase in oxygen free radicals and ROS production followed by oxidation and/or nitration of lipids, proteins, DNA, and carbohydrates. Therefore, chronically activated glia can kill adjacent neurons by the release of these highly toxic products.
Oxidative stress induces the release of zinc from MTs via NO, promoting the activity and expression of antioxidant enzymes, including MT-1/2 itself, thus reducing the oxidative damage and the consequences of the injurious stimulus.
MT-1/2 seems to be capable of triggering intracellular pathways associated with neuronal growth, survival and apoptosis, and perhaps even inflammation.
Autism
Heavy metal toxicity has been proposed as a hypothetical etiology of autism, and dysfunction of MT synthesis and activity may play a role in this. Many heavy metals, including mercury, lead, and arsenic have been linked to symptoms that resemble the neurological symptoms of autism. However, MT dysfunction has not specifically been linked to autistic spectrum disorders. A 2006 study, investigating children exposed to the vaccine preservative thiomersal, found that levels of MT and antibodies to MT in autistic children did not differ significantly from non-autistic children.
MT in Heart Disease
MT-IIA in heart-derived cell line in human confers oxidative protection. MT is a metal binding protein and cardio protective. In order to understand the molecular mechanisms underlying the role of MT in the heart a study established a stable MT-IIA over-expressing cardiac cell line, and evaluated its anti-oxidative property. The transfected cell line (H9c2MT7) exhibited similar growth kinetics and morphology. The western blotting analysis of this study showed that H9c2MT7 had a remarkable increase in MT protein level compared with the parent cell line H9c2. Transfection of MT conferred cellular resistance to cadmium toxicity and have established a stable human MT-IIA over-expressing cardiac cell line; and this cell line showed a markedly increased oxidative protection and would be useful for dissection of the mechanisms of MT in the cardiac protection.
MT and Diabetes
Apoptosis and Pathological Remodeling in the Diabetic Heart
A preclinical research in 2008 concluded the result that the acute angiotensin II administration to WT mice or neonatal cardiomyocytes increased cardiac apoptosis, nitrosative damage, and membrane translocation of the nicotinamide adenine dinucleotide phosphate oxidase (NOX) isoform p47phox. Prolonged administration of suppressor doses of Ang II (0.5 mg/kg every other day for 2 weeks) also induced apoptosis and nitrosative damage in both diabetic and non-diabetic WT hearts, but not in diabetic and non-diabetic MT-TG hearts. Long-term follow-up (1 to 6 months) of both WT and MT-TG mice after discontinuing Ang II administration revealed progressive myocardial fibrosis, hypertrophy, and dysfunction in WT mice but not in MT-TG mice. This study finalize MT suppresses Ang II-induced NOX-dependent nitrosative damage and cell death in both non-diabetic and a diabetic heart early in the time course of injury and prevent the late development of Ang II-induced cardiomyopathy.
MT, Zinc and Diabetes
Diabetes and polymorphisms in human genes control the cellular availability of zinc ions. One protein is the
zinc transporter ZnT-8 that supplies pancreatic β-cells with zinc. The other is MT 1A, a member of a protein
family that links zinc and redox metabolism. Changes in the availability of zinc ions modulate insulin signaling and redox processes. Both zinc and MT protect cells against the redox stress that occurs in diabetes and contributes to its progression towards diabetic complications, including heart disease.
Zinc an insulinomimetic
The MT in presence of zinc are able to reduce diabetic by insulinmimetic activity through phosphorylation thereby diabetic induced heart disease are also controlled. In diabetes, these functions of MT come to bear on insulin signaling and coronary heart disease. Insulin and zinc ions have potent stimulatory effects on lipogenesis and glucose uptake. Zinc-deficient animals are less sensitive to insulin. Zinc can replace insulin in mammalian cells cultured in serum-free media. The actions of zinc are intracellular because zinc increases the phosphorylation state of the insulin receptor, and hence, protein phosphorylation downstream in the insulin signaling pathways . It has been suggested that zinc inhibition of protein tyrosine phosphatase 1B, the major phosphatase controlling the phosphorylation state of the insulin receptor, is responsible for these insulinomimetic effects of zinc.
In mice, zinc supplementation prevents the development of diabetic cardiomyopathy through induction of MT, which has an antioxidant function in the heart.
REFERENCES
Metallothionein
The two faces of metallothionein in carcinogenesis: photoprotection against UVR-induced cancer and promotion of tumour survival, 2010.
Metallothioneins and zinc in cancer diagnosis and therapy,2012.
Neuroprotective and neuroregenerative properties of metallothioneins,2012.
Molecular functions of metallothionein and its role in hematological malignancies,2012.
Metallothionein and the biology of aging,2011.
Fluorescent probes for the structure and function of metallothionein,2009.
Metallothioneins in human tumors and potential roles in carcinogenesis,2003.
A Review of Metallothionein Isoforms and their Role in Pathophysiology,2011.
The human metallothionein gene family: structure and expression,1984.
Metallothionein Expression in Animals: A Physiological Perspective on Function,2000.
Metallothionein polymorphisms in pathological processes,2011.
The magic numbers of metallothionein,2011.
Mammalian metallothioneins: properties and functions,2011.
Clinical significance of metallothioneins in cell therapy and nanomedicine,2013.
Expression of functional metallothionein isoforms in papillary thyroid cancer.
Molecular and Cellular Endocrinology 2009
Interaction of dietary zinc and intracellular binding protein metallothionein in postnatal bone growth.
Bone 2009
Metallothionein protection of cadmium toxicity.
Toxicology and Applied Pharmacology 2009
Cadmium Concentration and Metallothionein Expression in Prostate
Cancer and Benign Prostatic Hyperplasia of Humans.
J Formos Med Assoc 2009
Metallothionein in the central nervous system: Roles in
protection, regeneration and cognition.
Neuro Toxicology 2008
Levin DEdward, Perraut Charles, Pollard Ninitia, Freedman HJonathan:
Metallothionein expression and neurocognitive function in mice.
Physiology & Behavior 2006
Protein tyrosine phosphatases as targets of the
combined insulinomimetic effects of zinc and oxidants.
BioMetals 2005
Matteo Maria Ottaviani