Description and Discovery


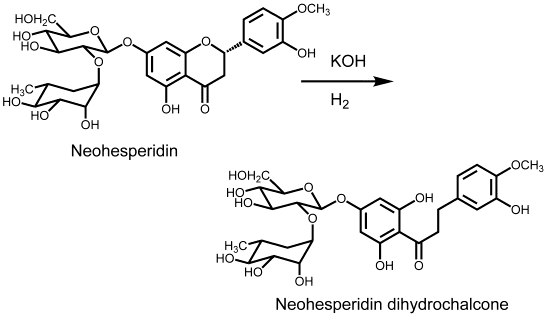
Neohesperidin dihydrochalcone, sometimes abbreviated to neohesperidin DC or NHDC, is a sweet substance of molecular weight 612.6 produced by alkaline hydrogenation (treatment with potassium hydroxide or another strong base, and then catalytic hydrogenation) of neohesperidin, which is a such bitter compound that can be isolated from the peel and pulp of orange, grapefruit, and other citrus fruit. It is a flavonoid and in pure form is found as an odorless white to pale yellow substance not unlike powdered sugar.
NHDC is commonly known as a non-nutritive sweetener and artificial sweetener approved for use in the European Union, but not approved by the FDA (so it can’t be used in the United States). Its potency as an intensive sweetener is naturally affected by such factors as the application it is used for and the pH of the product, however aqueous solutions of 0.0045% NHDC and 5% sucrose are approximately equisweet.
It has also the characteristics of a taste enhancer, so its addition to other sweeteners, such as saccharin, cyclamate and acesulfame-K increases the total perceived taste intensity of the mixture beyond the level predicted by the combination of the two substances.
It was first prepared by Horowitz and Gentili in 1963 as part of a United States Department of Agriculture research program to find methods for minimizing the taste of bitter flavorants in citrus juices.
(Neohesperidin Dihydrochalcone is Not a Taste Enhancer in Aqueous Sucrose Solutions, 2000)
(Antioxidant Properties of Neohesperidin Dihydrochalcone: Inhibition of Hypochlorous Acid-Induced DNA Strand Breakage, Protein Degradation, and Cell Death, 2006)
(Neohesperidin Dihydrochalcone, 2012)
Antioxidant Properties
In more recent studies NHDC showed remarkable radical scavenging activity against stable radical and reactive oxygen species (ROS) in concentration dependent manner. Especially, NHDC was the most potent inhibitor of H2O2 and HOCl. It showed HOCl scavenging activity of 93.5% and H2O2 scavenging property of 73.5%. Moreover, NHDC could inhibit protein degradation, plasmid DNA strand cleavage and HIT-T15, HUVEC cell death from HOCl attack.
NHDC's antioxidant activity is different against the different radicals:
Stable Radical (·ABTS+): using the Trolox equivalent antioxidant capacity (TEAC), it has an antioxidant activity greater than Trolox, showing that dihydrochalcone compounds have strong hydrogen donation capacity.

Superoxide Radical (·O2-): it has not a strong activity, however it's still stronger than other antioxidants like mannitol and BHT. NHDC and neohesperidin have several oxidizing groups and this result indicates that the oxidation group might contribute to superoxide radical scavenging activity.
Hydroxyl Radical (·OH): It has relatively lower scavenging effect on hydroxyl radicals rather than on the other ROS. But it can inhibit hydroxyl radical-induced degradation of deoxyribose into malonaldehyde in a concentration dependent manner.
Hydrogen Peroxide (H2O2): It has an high activity. Neohesperidin, phloridzin, and ascorbic acid have significant H2O2 scavenging capacity, and dihydrochalcone structure might have benefits to scavenge hydrogen peroxide.
Hypochlorous Acid (HOCl): very powerful scavenger with more than 90% of relative inhibition at 10mM. From this result, dihydrochalcone structure seems to have benefits to scavenge hypochlorous acid.
The possible mechanism of HOCl scavenging activity is chlorination. Recent data indicate that the reaction between flavonols and HOCl may be more complicated than a simple oxidant–antioxidant interaction, and that phenolic compounds can react with HOCl to form stable chlorinated components with each product potentially having a unique reactivity.

The antioxidant activity of flavonoids resides in the aromatic OH groups. In the ring B, this is the catechol moiety and the activity of one of the hydroxyl groups is enhanced by the electron donating effect of the other ones. In the AC-ring, the number of hydroxyl group is an important factor to show antioxidant activity and 2�-hydroxyl group of dihydrochalcone is the most important pharmacophore. Also 2,6 or 2,4,6-hydroxyacetophenone moiety is a unique pharmacophore responsible for the antioxidant activity of dihydrochalcones, which is quite different from the case of other flavonoids. Interestingly IC50 vale (concentration required for 50% inhibition) of NHDC on HOCl is the lowest and the highest on ·OH. NHDC has higher scavenging activity about hypochlorous acid than hydroxyl radical might be caused by its structural characteristics. So NHDC's potent inhibition of H2O2 and HOCl seems to be related to the dihydrochalcone structure, which are a family of the bicyclic flavonoids, defined by the presence of two benzenoid rings joined by a saturated three-carbon bridge. Moreover several chalcones inhibit various enzymes involved in the generation of reactive oxygen species.


These chemical characteristics against H2O2 and HOCl are reflected in biology with inhibition of protein degradation by inhibition of the ‘Fenton reaction’ (H2O2+ �Fe2�+→ ·OH�+OH-+
�Fe3+�)-induced protein degradation, inhibition of plasmid DNA strand breakage with more benefits for hypochlorous acid and inhibition of cellular damage preventing cell death, always without toxic side effects
(Antioxidant Properties of Neohesperidin Dihydrochalcone: Inhibition of Hypochlorous Acid-Induced DNA Strand Breakage, Protein Degradation, and Cell Death, 2006)
Potential Fields of Application
Reactive oxygen species (ROS) and free radicals induce membrane damages, DNA strand breaks, and protein alterations. Oxidative stress, induced by oxygen radicals, is believed to be a primary factor in various degenerative diseases as well as in the normal process of aging. The ROS are involved in the course of aging, cancer, cardiovascular disease, diabetes, neurodegenerative disease, osteoporosis, etc. Among the various reactive species, superoxide radical (·O2-
), hydroxyl radical (·OH), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) play important roles in the pathophysiologies as mentioned above, which make them potential targets for the chemotherapy of inflammatory diseases.
In the specific NHDC offers protective effect on HIT-T15 cell line of Syrian hamster β-cells against HOCl attack in concentration dependent manner while BHT, a well-known antioxidant, showed just a little protection activity. So it could prevent one of main mechanisms in the pathogenesis of diabetes mellitus which involves β-cell dysfunction derived from excessive oxidative stress and inflammation. In addiction it has been appreciated that inflammatory cells produce ROS and that these molecules are implicated in endothelial dysfunction associated with hypertension, atherosclerosis, and ischemia–reperfusion and NHDC demonstrated a protective effect on human endothelial cell death from HOCl attack. So this protective effect on cellular damage imply that the dihydrochalcones can be developed for anti-inflammatory drug. NHDC also showed a marked capacity to reduce the gastric ulcer index in cold-restraint induced ulcer in dose dependent manner.
NHDC can be metabolized by human intestinal bacteria, so anti-oxidative function might be limited in inflammatory gastro-intestinal diseases in vivo for oral administration. Therefore for the study about protective function of NHDC on ROS-induced toxicity and inflammation in vivo, i.p. or i.v. injection might be more profitable than oral administration.
(Antioxidant Properties of Neohesperidin Dihydrochalcone: Inhibition of Hypochlorous Acid-Induced DNA Strand Breakage, Protein Degradation, and Cell Death, 2006)
Metabolism and Toxicity
NHDC is a low-digestible substance, so its metabolism and absorption are dependent on the action of gut bacteria which convert neohesperidin dihydrochalcone anoxically to 3-(3-hydroxy-4-methoxyphenyl)propionic acid or 3-(3,4-dihydroxyphenyl)propionic acid. Two transient intermediates are identified as hesperetin dihydrochalcone 4'- β-d-glucoside and hesperetin dihydrochalcone. These metabolites suggest that neohesperidin dihydrochalcone is first deglycosylated to hesperetin dihydrochalcone 4'- β-d-glucoside and subsequently to the aglycon hesperetin dihydrochalcone. The latter is hydrolyzed to the corresponding 3-(3-hydroxy-4-methoxyphenyl)propionic acid and probably phloroglucinol. Eubacterium ramulus and Clostridium orbiscindens are not capable of converting neohesperidin dihydrochalcone. However, hesperetin dihydrochalcone 4'- β-d-glucoside is converted by E. ramulus to hesperetin dihydrochalcone and further to 3-(3-hydroxy-4-methoxyphenyl)propionic acid, but not by C. orbiscindens. In contrast, hesperetin dihydrochalcone is cleaved to 3-(3-hydroxy-4-methoxyphenyl)propionic acid by both species. The latter reaction is shown to be catalyzed by the phloretin hydrolase from E. ramulus.
(Studies in Natural Products Chemistry, Volume 39, 2013)
(Degradation of Neohesperidin Dihydrochalcone by Human Intestinal Bacteria, 2005)
There are no studies which demonstrates that it can start reactions of auto-oxidation like other molecules like Divicine, an aglycone of vicine and an oxidant with alkaloidal properties found in fava beans and Lathyrus sativus which auto-oxidizes rapidly at neutral pH, generating H2O2 by an O2-dependent chain mechanism (transiently inhibited by superoxide dismutase) creating a free-radical which oxidizes glutathione causing hemolysis known as “favism” in glucose-6-phosphate dehydrogenase deficient subjects who can't reduce the oxidized glutathione.
(Auto-oxidation of Dialuric Acid, Divicine and Isouramil. Superoxide Dependent and Independent Mechanisms, 1989)
(Divicine)
(Mechanism of Action of Divicine in a Cell-free System and in Glucose-6-phosphate Dehydrogenase-deficient Red Cells, 1984)
Other studies also demonstrates that providing an overall intake up to about 750 mg neohesperidin dihydrochalcone per kg body weight per day did not show any compound-related untoward effect in rats (Subchronic (13-week) oral toxicity of neohesperidin dihydrochalcone in rats, 1990) and no embryotoxic/teratogenic effects were observed at NHDC levels of up to 5% of the diet, the highest dose level tested, at which the rats consumed about 3.3g/kg body weight/day. (Embryotoxicity and teratogenicity study with neohesperidin dihydrochalcone in rats, 2004)
Conclusion
A molecule born as an artificial sweetener showed more interesting properties as the possibility of therapeutic effect of NHDC on ROS-related inflammatory diseases due to its scavenging activity not only for stable radical but also for various inflammation-related ROS, with a variable potency, depending on the assayed compound and reactive species.
NHDC has more inhibitory effect than ascorbic acid and BHT (known anti-inflammatory) in most assays and showed extensive inhibitory effect especially on non-radical ROS (H2O2, HOCl).
So its potent antioxidant activity combined with the low toxicity prospect a lot of medical implications.