DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)
External links not available on Wikipedia have to be added here
THE GENE
Wikigenes includes links to
- NCBI Gene
- NCBI SNP
- iHOP resource
- OMIM
- SNPedia
- UniProt
- Ensembl
- HGNC
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage
The Protein Aminoacids Percentage gives useful information on the local environment and the metabolic status of the cell (starvation, lack of essential AA, hypoxia)
Protein Aminoacids Percentage (Width 700 px)

SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
biological function
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC USE
TRPV1-4 AMMINOACIDS PERCENTAGE

TRPV (Wikipedia) (Transient Receptor Potential Vanilloid) is a family of transient receptor potential (TRP) ion channels.These channels are selective for calcium and magnesium over sodium ions. Like other members of the TRP superfamily, TRPV channels can be activated through seemingly disparate mechanisms. In vertebrates, several members of the family are sensitive to elevated temperature, making them thermoTRPs. The first member of this family that was isolated, TRPV1, is also sensitive to capsaicin, the pungent ingredient in "hot" chili peppers and accordingly TRPV1 is also sometimes referred to as the capsaicin or vanilloid receptor.
The number of TRPV channel variants differs between species. Worms have 5, flies only 2, mice and humans 6 distinct receptors. The TRPV receptors can also form heteromers that exhibit unique conductance and gating properties, further increasing their functional diversity.

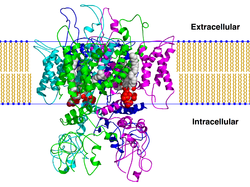
These channels are tetrameric in structure and they can be homo-tetrameric or hetero-tetrameric.
The four subunits are simmetrycally arranged around the pore.
The most recent research about heteromerization suggest that all four thermosensitive TRPVs(1-4)
can form heteromers with each other; but how TRPV subunits recognize and interact with each other is not yet understood.
The TRPV channel monomeric subunit components each contain six transmembrane ™ domains (designated S1–S6) with a pore domain between the fifth (S5) and sixth (S6) segments.
TRPV subunits contain three to five N-terminal ankyrin repeats.

Activation and Function
TRPV channels can be activated through a variety of mechanisms. TRPV1 to TRPV4 can act as thermometers on a molecular level. Interestingly, each of these channels has a different thermal threshold for activation: TRPV2 is activated at 52°C, TRPV1 at 43°C, TRPV3 at 33°C, and TRPV4 below 33°C.
This has not been proven in sensory neurons where for example no correlation between TRPV2 and heat sensitivity could be shown.
TRPV1 is essential for inflammatory thermal hyperalgesia.
The mammalian TRPV subfamily, named after the vanilloid receptor 1 (VR1, now TRPV1), contains six members, TRPV1-TRPV6. The N-terminus of each TRPV channel includes three to four ankyrin repeats, the C-terminus contains a TRP domain.
TRPV1-TRPV4, which share 40 to 50% amino acid homology, are temperature sensitive Ca2+-permeable, non selective cation channels. TRPV1, first identified as a receptor that binds capsaicin, is a relatively Ca2+-selective ion channel with an outwardly rectifying current-voltage relation and Ca2+-dependent desensitisation.
TRPV2 is ~50% identical to TRPV1 but is insensitive to capsaicin and low pH. It is activated by heat at a higher temperature threshold than TRPV1 (>52°C).
TRPV3 is temperature sensitive in the physiologic temperature range (31 to 39°C).
TRPV4, first described as a channel activated by hypotonicity-induced cell swelling, is also temperature sensitive, and activated by acid pH, mechanical stimuli and chemical compounds.
TRPV5 and TRPV6 are Ca2+-permeable TRP channels with a very high selectivity for Ca2+. KU Leuven
TRPV1 has a wide tissue distribution. High expression levels are observed in dorsal root ganglia, trigeminal ganglia, and nodose ganglia.
TRPV1 is predominantly expressed in neurons with small and medium diameters (mainly peptidergic neurons; C-fibers) that are important in the development of neurogenic pain and inflammation.
and to a lesser extent in non-peptidergic neurons (Aδ-fibers) that play a critical role in mediating chronic and mechanical pain.
TRPV1 have important function in homeostasis,for example, in thermoregulation.
TRPV1 is a nonselective cation channel that may be activated by a wide variety of exogenous and endogenous physical and chemical stimuli. The best-known activators of TRPV1 are: temperature greater than 43°C, acidic conditions, capsaicin, the pungent compound in hot chilli peppers, allyl (garlic) isothiocyanate,topical analgesic camphor, the pungent compound in mustard, wasabi and black pepper.The activation of TRPV1 leads to a painful, burning sensation. Its endogenous activators include: low pH (acidic conditions), the endocannabinoid anandamide, and N-arachidonoyl-dopamine. TRPV1 receptors are found mainly in the nociceptive neurons of the peripheral nervous system, but they have also been described in many other tissues, including the central nervous system. TRPV1 is involved in the transmission and modulation of pain (nociception), as well as the integration of diverse painful stimuli.
For more informations: TRPV1 Capsaicine

It is 50% identical to TRPV1 and forms a weakly Ca2+ -selective cation channel. It is activated by temperatures >52°C when expressed in Xenopus laevis oocytes. There are, however, species-dependent differences in this activation, and human TRPV2 is apparently not activated by.
TRPV2 is reported to be present in a wide variety of tissues, including brain, pancreas, spleen, lung, stomach, intestine, bladder, prostate, and blood cells. It has been proposed that TRPV2 may serve as an endosomal calcium release channel that controls endosome fusion and/or exocytosis. Indeed, many studies suggest that activation of TRPV2 causes translocation of the channel to the plasma membrane. It is noteworthy that aberrant localization of TRPV2 is detected in rodent models of muscular dystrophy, and expression of dominant-negative TRPV2 reduced muscle damage . TRPV2 has been shown to be expressed in macrophages and has a critical role in macrophage particle binding and phagocytosis. TRPV2−/− mice have been consistently shown to be more vulnerable when challenged with pathogens such as Listeria monocytogenes, mainly because of the greater organ bacterial load.
In this study, we found that the TRPV2 receptor is frequently expressed in pulpal sensory neurons relative to their expression in trigeminal ganglia as a whole. By contrast, TRPV1 is underrepresented in pulpal sensory neurons relative to their expression in the trigeminal ganglia and in the periodontal tissue. These observations support the contention that the dental pulp is a uniquely innervated nociceptive tissue and may, in fact, have a limited capacity to detect heat viaTRPV1, at least under uninjured conditions. This finding could be related to the clinical observation that heat is an unreliable stimulus to test pulp vitality.
Results suggest either that very few TRPV1 afferents are required to mediate heat sensitivity of the DP, or that as-yet-unidentified heat transducers or mechanisms are involved. TRPV1-expressing pulpal neurons take on a more critical role after injury, namely, in the setting of pulpal inflammation and neural degeneration.
Given the high expression of TRPV2 in the dental pulp and the fact that this receptor is unlikely to contribute to heat detection, it is of great interest to determine its function in the DP. One intriguing possibility is that TRPV2 receptors are expressed on mechano-sensitive nociceptors, as recently demonstrated in an electrophysiological study of somatic afferents. Since the dental pulp is densely innervated with mechano sensitive nociceptive fibers, and TRPV2 is concentrated in myelinated afferents, it is conceivable that the two populations overlap, and that TRPV2 contributes to the mechano-sensitivity of the pulpal afferent.
From this analysis, we conclude that afferents that innervate dental pulp and periodontal tissues have a neurochemical signature manifest in the differential expression of the heat-sensitive channel TRPV1 and the purported heat-/mechano-sensitive channel TRPV2. The expression pattern of these receptors in peripheral afferents of these tissues likely contributes to the high sensitivity of these tissues to detection of innocuous as well as noxious thermal and mechanical stimuli.
TRPV3 also forms a voltage-sensitive weakly Ca2+-selective cation channel that is activated by warm temperatures (33–39°C) and a variety of botanical compounds including camphor, eugenol, thymol, and carvacrol. In rodent skin keratinocytes, TRPV3 is proposed to sense warmth; TRPV3−/− mice display altered behavioral responses to heat, including altered temperature preferences in thermotaxis assays. Unexpectedly, a recent study found that TRPV3 is required for epidermal growth factor receptor signaling in keratinocytes, and TRPV3−/− mice exhibit wavy hair coat and curly whiskers. Many pro-inflammatory agents such as bradykinin, histamine, ATP, and prostaglandin E2 sensitize TRPV3 function. ATP interacts with the channel's N-terminal ankyrin repeats to regulate this sensitization. Elevated TRPV3 activity can dramatically influence skin integrity; rodents with constitutively active TRPV3 channels have an increased susceptibility to dermatitis and skin lesions.

TRPV4 is activated by warm temperatures in the range of 27–34°C; consequently, at physiological temperatures, the channel should demonstrate significant constitutive activity. TRPV4 is sensitive to osmotic and mechanical stimuli, such as cell swelling or fluid flow, and sensitivity of TRPV4 to these stimuli may depend on phospholipase A2 activation and the subsequent production of the arachidonic acid metabolite epoxyeicosatrienoic acid (EET). TRPV4 can also be activated by botanical and synthetic compounds such as 4α-phorbol-12,13-dihexanoate, bisandrographolide. A variety of kinases also seem to modulate its activity.
TRPV4 is widely distributed and was proposed to sense temperature in the hypothalamus, skin and primary sensory neurons. The sensitivity of TRPV4 to osmotic stimuli may be important for cellular and systemic osmoregulation. TRPV4 was detected in putative osmoreceptive neurosensory cells around the ventricle and TRPV4−/− mice display diminished drinking, elevated systemic osmotic pressure, and reduced synthesis of antidiuretic hormone in response to systemic hypertonicity induced by salt ingestion.TRPV4 may also contribute to the development of mechanical hyperalgesia after inflammation and injury. In support of this hypothesis, TRPV4−/− mice display impaired bladder function. TRPV4 seems to regulate vascular tone and bone deposition and remodelin. It is noteworthy that mutations in TRPV4 have been identified in patients with three dominantly inherited skeletal phenotypes: autosomal-dominant brachyolmia, spondylometaphyseal dysplasia Kozlowski type, and metatropic. TRPV4 mutations have also been linked to patients with congenital distal spinomuscular atrophy, Charcot-Marie-Tooth disease type 2C, and scapuloperoneal spinal muscular atrophy.
At our body surface, the epidermis absorbs UV radiation. UV overexposure leads to sunburn with tissue injury and pain. This fact is caused by a nonselective cation channel higly expressed in epithelial skin cells: TRPV4. After an UVB exposure, mice with induced Trpv4 deletions, specifically in keratinocytes, are less sensitive to noxious thermal and mechanical stimuli than control animals. In culture, where mice are treated with a TRPV4-selective inhibitor, damage and epidermal tissue damage and expression of endoteli-1 decrease. In humans, sunburn enhances epidermal expression of TRPV4 and endothelin-1, underscoring the potential of keratinocyte-derived TRPV4 as a therapeutic target for UVB-induced sunburn, in particular pain.
Epidermis provides barrier protection against dehydration and the potentially harmful
external environment. Accordingly, skin is the site of first interaction between ambient environment and immunologically competent organismal structures. Sensory neurons in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) are endowed with sensory transduction capacity for heat, cold, mechanical cues, itch, and pain, and their axons directly interface with skin epithelium.
Skin tissue injury caused by UVB has been elucidated to be mediated by cytokines and chemokines:
which are also known to cause and facilitate pain.
Using genetically engineered mice and cultured skin epithelial cells, it has been identified that the calcium permeable TRPV4 ion channel as critical for translating the UVB stimulus into intracellular signals and also into signals from epithelial skin cell to sensory nerve cell that innervates the skin, causing pain.
After an UVB exposure, mice with induced Trpv4 deletions, specifically in keratinocytes, are less sensitive to noxious thermal and mechanica stimuli than control animals. Exploring the mechanism , it has been observed that epidermal TRPV4 orchestrates UVB-evoked skin tissue damage and increased expression of the proalgesic/algogenic mediator endothelin-1. UVB triggers TRPV4 channel activation, which leads to Ca2+ influx into keratinocytes. In turn, this transforms the cells to function in a proalgesic manner.
The team identified PLC as a key player, necessary for production of high-activity smallmolecule intermediaries such as IP3. ET1–ET signaling, which activates PLC, is also essential for Ca2+ signaling by epidermal TRPV4, and here, ET1–ET® seem to function in an autocrine/paracrine feed-forward loop.
Epidermal TRPV4 is absolutely needed for injected ET1 to function in a proalgesic manner.
Endothelins act as a potent inducer of melanocyte differentiation. Given the finding that epidermal TRPV4 regulates ET1 secretion, TRPV4 becomes poised not only to orchestrate the acutesentinel function of keratinocytes, which induces pain and hypersensitivity, which leads to protective temporary rest and nonuse, but also to provide longer-term protection, by iperpigmentation.
TRPV4 could be a prime therapeutic target for treatments aimed at alleviating the pain, tissue damage, and skin blistering associated with sunburn.
In conclusion, activation of TRPV4 in skin by UVB, evokes sunburn pain, highlighting the forefront-signaling role of the skin and TRPV4.
TRPV5 is expressed in a number of tissues. In the kidney, TRPV5 is predominantly expressed in the distal convoluted and connecting tubule where it is important for transcellular transport and active reabsorption of Ca2+ in the kidney. Indeed, ablation of the TRPV5 results in impaired Ca2+ resorption in the distal convoluted and connecting tubule. TRPV5−/− mice excrete approximately six times more Ca2+ in their urine and display compensatory increases in vitamin D levels and intestinal hyperabsorption of Ca2+. In addition, they display polyuria with significantly more acidic urine than that of wild-type mice. TRPV5−/− mice also display bone abnormalities, including reduced trabecular and cortical bone thickness and increased osteoclast number and size. Yet TRPV5−/−
mice had low serum deoxypyridinoline levels, indicating decreased rate of bone breakdown and, unlike other mouse models with decreased osteoclast function, showed decreased bone thickness without osteopetrosis.
TRPV6 is more widely distributed than TRPV5. In the intestine, TRPV6 localizes to the brush border membrane of enterocytes, where it is proposed to mediate transcellular Ca2+ entry. Indeed, TRPV6−/− mice that were fed a low Ca2+ diet exhibited decreased Ca2+ absorption and serum Ca2+levels compared with wild-type mice; however, a disruption of closely adjacent EphB6 gene in the TRPV6−/− mice may complicate the interpretation of this phenotype. In the kidney, TRPV6 is expressed in the convoluted tubules, connecting tubules, and cortical and medullary collecting ducts of the nephron, where it helps resorb Ca2+ . In the placental trophoblast, TRPV6 contributes to the transfer of Ca2+ from mother to fetus and may contribute to the reduced litter size of TRPV6−/− mice.

by Wilfred Soldati