An acetylcholine receptor (abbreviated AChR) is an integral membrane protein that responds to the binding of acetylcholine, a neurotransmitter.



Muscarinic R

M Receptors subtypes
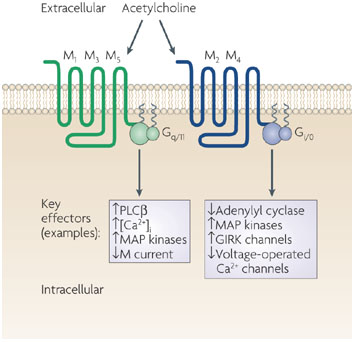

Inositol
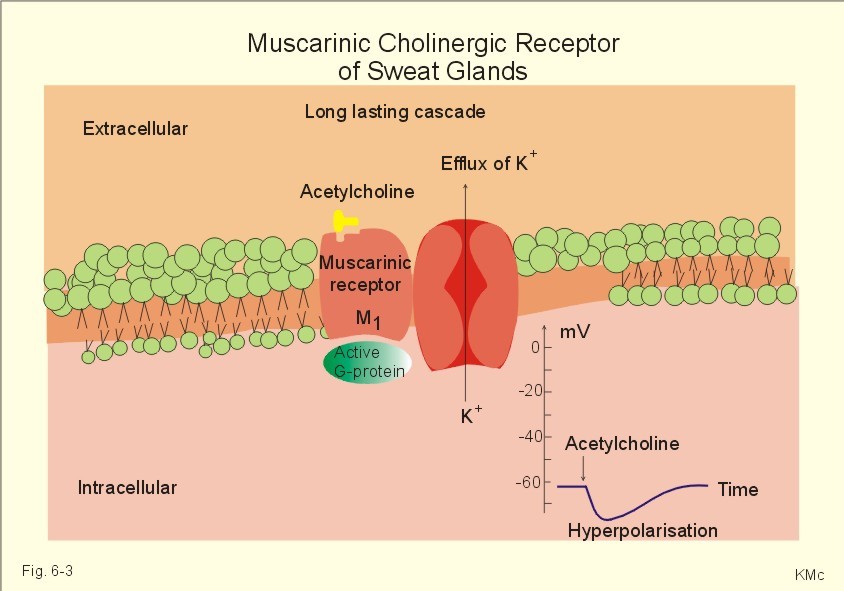
MUSCARINIC RECEPTOR SUBTYPES IN THE ALIMENTARY TRACT
Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor , 2013
- Abstract: Acetylcholine is an important neurotransmitter whose effects are mediated by two classes of receptors. The nicotinic acetylcholine receptors are ion channels, whereas the muscarinic receptors belong to the large family of G protein coupled seven transmembrane helix receptors. Beyond its function in neuronal systems, it has become evident that acetylcholine also plays an important role in non-neuronal cells such as epithelial and immune cells. Furthermore, many cell types in the periphery are capable of synthesizing acetylcholine and express at least some of the receptors. In this review, we summarize the non-neuronal functions of the muscarinic acetylcholine receptors, especially those of the M2 muscarinic receptor in epithelial cells. We will review the mechanisms of signaling by the M2 receptor but also the cellular trafficking and ARF6 mediated endocytosis of this receptor, which play an important role in the regulation of signaling events. In addition, we provide an overview of the M2 receptor in human pathological conditions such as autoimmune diseases and cancer.
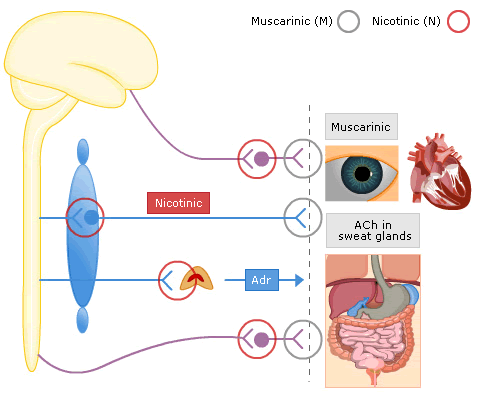
Nicotinic Receptors
These are found in the sympathetic ganglia and the motor end-plates of skeletal muscle
Nicotinic receptors are ligand-gated ion channel receptors and their actions are mimicked by nicotine, but unaffected by atropine


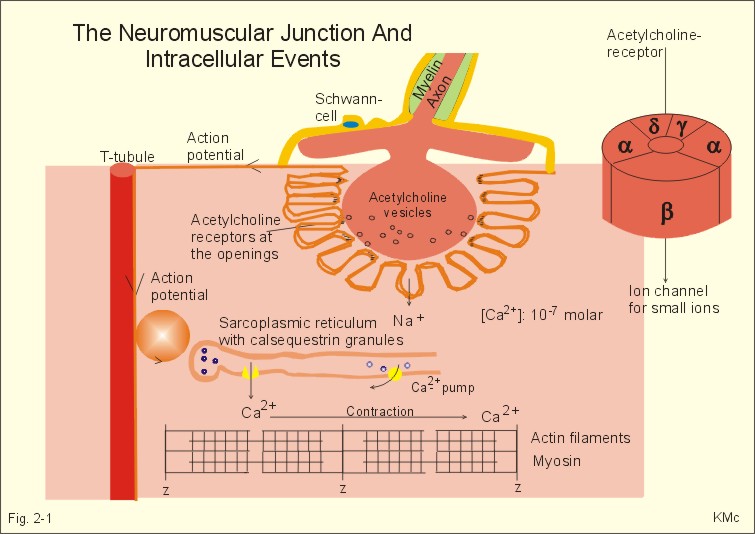
Cholinergic synapse
The Neuromuscular Junction
Pharmacology of cholinergic receptors
