(Greek: "big eaters", makros = large, phagein = eat) are cells within the tissues that originate from specific white blood cells called monocytes.
Macrophage functions
Their role is to phagocytize (engulf and then digest) cellular debris and pathogens and to stimulate lymphocytes and other immune cells to respond to the pathogen.

Macrophages activation
Retinoid X receptors in macrophage biology, 2013
NADPH oxidase complex generates a respiratory burst. Superoxide can react to form hydrogen peroxide and hypochlorus acid, which together participate in bacterial killing.
Role of NADPH-Oxidase
NADPH-Oxidase activation requires Rac translocation to the membrane that is mediated by geranylgeranylation.
Nuclear Factors involved in MA.PDF
Nuclear Factors involved in MA, Abstract
Lysosomes acidification
Macrophage cytoplasmic vesicle pH gradients and vacuolar
H1-ATPase activities relative to virus infection 1998
Macrophages behavior bifurcation 
Top figure: Strong lysosomes acidification, foreign antigens full digestion

Bottom figure: Poor lysosomes acidification, foreign antigens partial digestion and antigen presentation
Dendritic Cells

IL-6 and TNF alpha production by macrophages 2007
Macrophages function as a ferritin iron source for cultured human erythroid precursors 2007
Bacterial Inhibitors of V-ATPase
Macrophages Polarization
Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression1 2006
Macrophages were obtained by culturing monocytes (98% CD14+, 13% CD16+) for 7 days in RPMI 1640 (Biochrom) supplemented with 20% FCS (HyClone) and 100 ng/ml M-CSF in FCS-coated dishes at a density of 1.5 × 105/cm2. Macrophage polarization was obtained by removing the culture medium and culturing cells for an additional 18 h in RPMI 1640 supplemented with 5% FCS and 100 ng/ml LPS plus 20 ng/ml IFN-{gamma} (for M1 polarization) or 20 ng/ml IL-4 (for M2 polarization).
- Monocytes and tissue macrophages provide both immediate defense against foreign agents and assist during the setting off and development of the adaptive immune response. Monocytes originally derive from CD34+ myeloid progenitor cells in the bone marrow, circulate in the bloodstream, and enter peripheral tissues where they mature into different types of resident macrophages, characterized by low oxygen consumption, low protein synthesis rate, and modest cytokine production.
Inflammation due to tissue damage or infection results in resident macrophage activation, which increases the production of cytokines, chemokines, and other inflammatory mediators, as well as monocyte recruitment. In the context of specific immune response, the cytokine milieu compels mononuclear phagocytes to express specialized and polarized functional properties.
- Mirroring the Th1/Th2 nomenclature, many refer to polarized macrophages as M1 and M2 cells.
- Classically polarized activated M1 macrophages have long been known to be induced by IFN-γ alone or in concert with microbial stimuli as LPS, or cytokines as TNF and GM-CSF. M1 cells have an IL-12high, IL-23high, IL-10low phenotype, are proficient producers of effector molecules (reactive oxygen and nitrogen intermediates) and inflammatory cytokines (IL-1β, TNF, IL-6), contribute as inducer and effector cells in polarized Th1 responses, and mediate resistance against intracellular parasites and tumors
- M2 form of macrophage activation is a generic name used for various forms of nonclassically activated macrophages resulting from cell exposure to IL-4 or IL-13, immune complexes, IL-10, glucocorticoid, or secosteroid (vitamin D3) hormones. The various forms of M2 macrophages share an IL-12low and IL-23low phenotype, generally display high levels of scavenger, mannose, and galactose-type receptors, and arginine metabolism is shifted to production of ornithine and polyamines via arginase.

CD19+
cd19+ cells suppression
cd19+ cells suppression and IDO
A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation, 2005
Abstract
By ligating CD80/CD86 (B7) molecules, the synthetic immunomodulatory reagent CTLA4-Ig (soluble synthetic CTLA4 fusion protein) induces expression of the enzyme indoleamine 2,3-dioxygenase (IDO) in some dendritic cells (DCs), which acquire potent T cell regulatory functions as a consequence. Here we show that this response occurred exclusively in a population of splenic DCs co-expressing the marker CD19. B7 ligation induced activation of the transcription factor signal transducer and activator of transcription (STAT1) in sorted CD19+, but not CD19NEG, DCs. STAT1 activation occurred even when DCs lacked receptors for type II IFN (IFNγ); however, STAT1 activation and IDO up-regulation were not observed when DCs lacked receptors for type I IFN (IFNαβ). Thus, IFNα, but not IFNγ, signaling was essential for STAT1 activation and IDO up-regulation in CD19+ DCs following B7 ligation. Consistent with these findings, B7 ligation also induced sorted CD19+, but not CD19NEG, DCs to express IFNα. Moreover, recombinant IFNα induced CD19+, but not CD19NEG, DCs to mediate IDO-dependent T cell suppression, showing that IFNα signaling could substitute for upstream signals from B7. These data reveal that a minor population of splenic DCs expressing the CD19 marker is uniquely responsive to B7 ligation, and that IFNα-mediated STAT1 activation is an essential intermediary signaling pathway that promotes IDO induction in these DCs. Thus, CD19+ DCs may be a target for regulatory T cells expressing surface CTLA4, and may suppress T cell responses via induction of IDO.



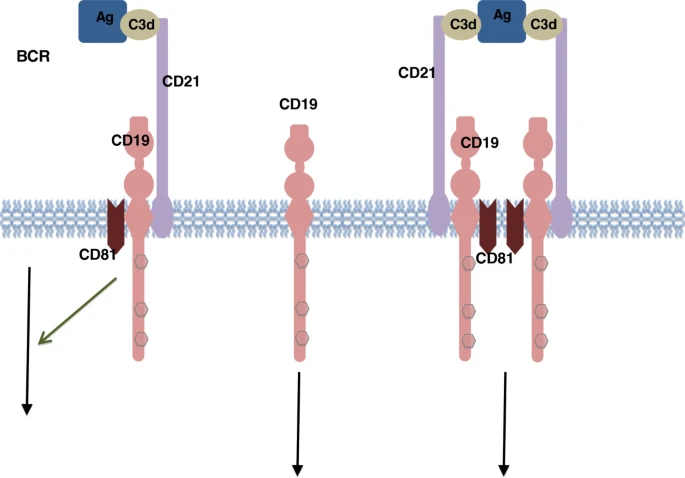
CD19: a biomarker for B cell development, lymphoma diagnosis and therapy, 2012