DESCRIPTION OF THE COMPOUNDS:
The Vinca alkaloids are a subset of drugs, called spindle inhibitors, that are derived from the periwinkle plant, Catharanthus roseus (also Vinca rosea, Lochnera rosea, and Ammocallis rosea). It is also commonly called the Madagascar periwinkle or the rose periwinkle. While they has been historically used to treat numerous diseases, they have most recently been employed for their anti-cancer properties. Actually, they are composed by synthetic or semisynthetic azotated bases.

MOLECULAR MECHANISM:
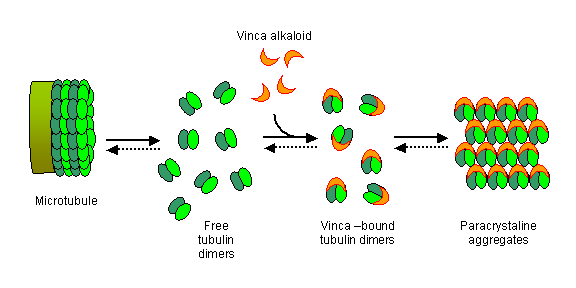
These agents do not work to alter DNA structure or function. These drugs interfere with the mechanics of cell division. During mitosis, the DNA of a cell is replicated and then divided into two new cells. The process involves spindle fibers, which are constructed with microtubules (formed by long chains of smaller subunits of proteins called tubulins).
Without functional spindle fibers or spindles, the cell cannot divide and will eventually die. So, the spindle inhibitor drugs function in a cell-cycle dependent manner.
Microtubules as a target for anticancer drugs, 2004.
Microtubulin binding sites as target for developing anticancer agents, 2004.
Vinca alkaloids inhibit cell proliferation by binding to microtubules, which leads to a mitotic block and apoptosis. Vincristine and related compounds induce destabilization of microtubules by binding to tubulin and blocking the polymerization. Exposure of cells to vinca alkaloids results in an induction of tumor protein p53 and cyclin-dependent kinase inhibitor 1A (p21) and in rapid alterations of protein kinase activities.
The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review, 1999.
These protein kinases are directly or indirectly responsible for Bcl-2 phosphorylation, which results in the inactivation of BCL-2 function.
Inactivation of Bcl-2 by phosphorylation,1995.
The induction of BCL-2 phosphorylation is followed by the loss of its ability to form heterodimers with BAX. The loss of BCL-2 function together with the elevations of p53 and p21 lead to apoptosis.
They also play a competitive role in the transport of amino acids in the intracellular compartment, in the inhibition of purine, of DNA and RNA, inhibition of protein synthesis and in the release of histamine from mast cells. They can also crystallize in the cell causing direct mechanical damage.
They do not selectively inhibit cancer cell division.They can also halt the division of some healthy cells, leading to some of the common side effects.
MITOSIS;
TUBULIN FILAMENTS;
BCL2;
P53;
CLASSIFICATION:
There are five major vinca alkaloids in clinical use:
-VINCRISTINE
-VINBLASTINE
-VINDESINE
-VINORELBINE
-VINFLUVINE
Vinca Alkaloids-chemical CTD
STRUCTURE:
Vinca alkaloids have a common chemical structure consisting of an asymmetric dimer composed of a vindoline ring connected to a catarantine ring through carbon-carbon bonds.
The discovery of the vinca alkaloids-chemotherapeutic agents against cancer, 1990.

Synthesis of the Vinca alkaloids
SOMMINISTRATION:
All vinca alkaloids are administered intravenously (IV), in rapid bolus or with short infusion and require special attention because they are vessels-sclerotics.
METABOLISM:
Vinca alkaloids are characterized by a large total distribution volume, rapid total plasma clearance and a long terminal half-life. After injection, they are eventually metabolized by the liver. It is therefore essential to monitor liver function and, if necessary, reduce the dose up to 50%. In humans the main elimination route of vinca alkaloids is via faecal excretion.
Pharmacokinetics and metabolism of vinca alkaloids, 1993.
Vinca alkaloids pharmacokinetics are time- and dose-dependent and show large inter- and intraindividual variability .
Pharmacokinetics and metabolism of vinca alkaloids, 1993.
Human liver microsomal cytochrome P450 3A isozymes mediated vindesine biotransformation. Metabolic drug interactions, 1993.
Vincristine: Can its therapeutic index be enhanced?, 2009.
CYTOCHROME P450;
TOXICITY:
It is characteristic of each drug belonging to the class. However, the vinca alkaloids have the common ability to induce thrombocytopenia, due to the abundance of tubulin which is present in thrombocytes.
Other common toxic effects of vinca alkaloids are:
• Hematologic (VBL > VCR)
• Neurologic (VCR > VBL)
• Gastrointestinal
• Endocrine (VCR > VBL)
• Dermatologic
• Cardiovascular
• Polmonary (VBL)
Moreover, vinca alkaloids commonly cause reversible peripheral neurological side effects, which include tingling of the fingers or toes, abdominal pain, constipation, and muscle weakness. Other side effects include bone marrow suppression and reversible hair loss. There may also be pain and irritation at the injection site.
RESISTANCE:
resistance mechanisms are common to all vinca alkaloids and are related to the alteration of tubulin molecules and to the overexpression of Pg-170. In particular, these mechanisms are:
• alterations of α-and / or β-tubulin
- Production of β-tubulin forms that have alterations in the binding site for the drugs;
- Production of α-and / or β-tubulin forms with altered level of the binding sites for GTP
• changes in the intracellular storage
- overexpression of P-glycoprotein
- overexpression of MRP-1
P-GLYCOPROTEIN;
ABCB1 is implicated in the resistance and pharmacokinetics of vinca alkaloids.
Molecular pharmacokinetics of catharanthus (vinca) alkaloids, 2007. ABCB1 mediates the biliary excretion of vinblastine and vincristine on the apical side of hepatocytes.
ABCB1, ABCC1, ABCC2, ABCC3, ABCC10, RALBP1 have been reported in association with vincristine resistance.
Vincristine transcriptional regulation of efflux drug transporters in carcinoma cell lines, 2006.
RALBP1/RLIP76 mediates multidrug resistance, 2007.
ABC TRANSPORTERS;
MULTI DRUG RESISTANCE;
To circumvent the problem of vinca alkaloid resistance, VINFLUNINE, a synthetic vinca alkaloid, has been developed.
Novel anti-tubulin cytotoxic agents for breast cancer, 2009. Vinflunine has shown superior preclinical antitumor activity, and displays a different pattern of resistance, compared with other agents in the vinca alkaloid class.
Wikipedia-Vinflunine;
VINCRISTINE
(Oncovin®, Vincasar PFS®, Vincrex®):
Also known as Leurocristine, it is used to treat many different types of cancer. It has the best affinity for tubulin compared to the other Vinca alkaloids.
It's the vinca alkaloid with longer terminal half-life and lower clearance.
Does not pass the blood-brain barrier.
Being carcinogenic, mutagenic and teratogenic, it causes mainly peripheral neurotoxicity, gastrointestinal toxicity (more rarely), but mild myelosuppression.
Myelosuppression may be severe in races with mutations in the gene coding for the p-glycoprotein (requires a reduction of dose).

Representation of the genes involved in the metabolism, transport, and downstream effects of the vinca alkaloid vincristine.
Structure of the Vincristine

PHARMACOKINETIC→
- high protein binding (75%)
- hepatic metabolism
- biliar (90%) and renal (10%) excretion
- half-life: from 19 to 155 hours
Wikipedia-Vincristine;
INDICATIONS:
It is frequently used in combination with other drugs. Malignancies in which vincristine is used include:
• acute leukemia
• rhabdomyosarcoma
• neuroblastoma
• Hodgkin's disease and other lymphomas
• lymphorecticular neoplasms
• childhood leukemias
SIDE EFFECTS:
Unlike some of the other vinca alkaloids, vincristine does not cause severe bone marrow suppression (decreased blood cell counts). Common side effects include:
• hair loss
• pain/redness at location of injection
• nausea/stomach pain/vomiting
• lowered blood cell count
• numbness
• headache
• constipation
• nervous system problems such as neuropathy or sensory impairment
• blurred or double vision
• back pain
• overall weakness
PHARMACOLOGICAL INTERACTIONS:
This drug should not be taken by a patient who is pregnant, planning a pregnancy, or breast-feeding as it may cause birth defects. Patients should not receive any immunizations (vaccinations) while taking this medication. Vincristine may cause immunosuppression (weakened immunity) and vaccinations could lead to an illness. Patients should notify their clinician about any prescription drugs taken concurrently with the chemotherapy and any other medical conditions, such as, chickenpox, herpes zoster infection, gout, kidney stones, infections, liver disease, nerve or muscle disease.
The vinca alkaloids: a new class of oncolytic agents, 1963.
Drugs know to potentiate vincristine-induced neurotoxicity through inhibition of CYP3A enzymes or inhibition of ABCB1, include nifedipine, cyclosporine, itraconazole, and voriconazole.
Vincristine: Can its therapeutic index be enhanced?, 2009.
Carbamazepine and phenytoin as CYP3A inducers increase the clearance of vincristine. Cytochrome P450-inducing antiepileptics increase the clearance of vincristine in patients with brain tumors, 1999.
VINBLASTINE
(Velban®, Velsar®):
Initially named vincaleukoblastine, VLB, it differs from Vincristine only for one CH3 group at the vindoline ring and has similar pharmacokinetic characteristics. It can also inhibit angiogenesis.
The main toxicity is non-cumulative myelosuppression, and then there is gastrointestinal toxicity, while neurotoxicity is rare.
ANGIOGENESIS;
Structure of the Vinblastine
PHARMACOKINETIC→
- hepatic metabolism (CYP3A4-mediated)
- biliar and renal excretion
- hal-life: 24.8 hours
Wikipedia-Vinblastine;
Vinblastine is metabolized to desacetilvinblastine and this compound is more biologically active than the parent drug.
Molecular pharmacokinetics of catharanthus (vinca) alkaloids, 2007.
INDICATIONS:
Cancers for which vinblastine is used include breast cancer, testicular cancer, lymphomas.
SIDE EFFECTS:
Common side effects include:
• bone or muscle pain
• nausea and vomiting
• alopecia
• sores in mouth and on lips
• swelling of feet or lower legs
Less common side effects include: dizziness, double vision, drooping eyelids, headache, jaw pain, mental depression, numbness or tingling in fingers and toes, pain finger and toe pain, testicle pain, waking difficulties, weakness, blood in urine or stools, and unusual bleeding or bruising.
Microtubules as a target for anticancer drugs, 2004.
Inhibition of cell migration and cell division correlate with distinct effects of microtubule inhibiting drugs, 2010.
VINDESINE
(Eldisine®, Fildesin®):
It is a semi-synthetic derivative of vinblastine.
Structure-activity relationships of dimeric Catharanthus alkaloids: Deacetylvinblastine amide (vindesine) sulfate, 1978.
Structure of the Vindesine

PHARMACOKINETIC→
- high protein binding (65-75%)
- hepatic metabolism (CYP3A4-mediated)
- biliar and renal excretion
- half-life: 24 hours
Wikipedia-Vindesine;
INDICATIONS:
Cancers for which vindesine is used include:
• acute lymphocytic leukemia
• lung carcinomas
• breast cancer
• chronic myelogenous leukemia
• colorectal cancer
SIDE EFFECTS:
Common side effects include:
• hair loss
• rash
• nausea and vomiting
• constipation
• stomach cramps
• jaw pain
• vein inflammation (phlebitis)
• decreased blood cell counts (also called bone marrow suppression or, myelosuppression)
• decrease in blood platelet number (thrombocytopenia)
www.macmillan.org.uk;
VINORELBINE
(Navelbine®) is a relatively new vinca alkaloid discovered in the mid-1990s by the Pierre Fabre Company. It is a semi synthetic derivative of vinblastine. It is unique among the alkaloids because it has a very liposoluble structure, which ensures the complete depolymerization of microtubules. It can also be administered orally.
Structure of the Vinorelbine

PHARMACOKINETIC→
- bioavailability: 43 +/- 14 (oral)
- protein binding: 79-91
- hepatic metabolism (CYP3A4-mediated)
- fecal (46%) and renal (18%) excretion
- half-life: 27.7 - 46.3 hours
Wikipedia-Vinorelbine;
The major metabolite is the desacetylvinorelbine.
Vinorelbine is metabolized to its active metabolite, 4-O-deacetyl vinorelbine and two other minor metabolites, 20'-hydroxyvinorelbine and vinorelbine 6'-oxide.
High performance liquid chromatography method for vinorelbine and 4-O-deacetyl vinorelbine: a decade of routine analysis in human blood, 2007.
New sensitive liquid chromatography method coupled with tandem mass spectrometric detection for the clinical analysis of vinorelbine and its metabolites in blood, plasma, urine and faeces, 2001.
In-vitro data in human hepatic microsomes indicated that CYP3A4 is the main enzyme involved in the hepatic metabolism of vinorelbine.
Characterization of human cytochrome P450 isoenzymes involved in the metabolism of vinorelbine, 2005.
CYP3A4 is mainly responsibile for the metabolism of a new vinca alkaloid, vinorelbine, in human liver microsomes, 2000.
INDICATIONS:
Cancers for which vinorelbine is used include:
• breast cancers
• testicular cancers,
• epithelial ovarian cancers
• non-small cell lung cancers
SIDE EFFECTS:
The side effects and toxicity of vinorelbine are generally milder than other vinca alkaloids. However, various side effects do frequently occur with treatment.
Common side effects include:
• myelosuppression, which results in a reduced number of platelets, red blood cells and white blood cells
• shortness of breath
• constipation or loose stools
• alopecia
• numbness in the hands or feet
Birth defects are possible so patients who are pregnant or considering becoming pregnant should not take this drug. Breast-feeding should also be avoided while using vinorelbine. It is also critical that no immunizations are administered while taking vinorelbine. As a result of a lowered number of white blood cells, the body's natural defense mechanisms are weakened and often unable to fight infection. Likewise, it is important to avoid interaction with individuals recently given an oral polio vaccination.
Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors, 2001.