DESCRIPTION
Ketamine is a drug used in human and veterinary medicine. Commercial brands of ketamine include Ketalar, Ketaset, Ketmex, Ketotal, Ketamine-500 (Astrapin) and Imalgen.
Pharmacologically, ketamine is classified as an NMDA receptor antagonist, although it can bind to opiod u receptors at high doses and can interact with muscarinic receptors.
Ketamine has a wide range of effects in humans, including analgesia, anesthesia, hallucinations, elevated blood pressure, and bronchodilation. It is primarily used for the induction and maintenance of general anesthesia, usually in combination with a sedative. Other uses include sedation in intensive care, analgesia (particularly in emergency medicine), and treatment of bronchospasm.
CHEMISTRY
The most common commercial preparation is a racemic mixture of two enantiomers, S(+) ketamine and R(–) ketamine. S(+) ketamine has four times the affinity of R(–) ketamine for the NMDA receptor. and also binds to mu and kappa opioid receptors.

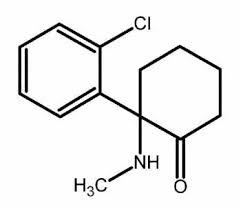
Ketamine, 2-(o-chlorophenyl)-2-(2-methylamino)cyclohexanone, is synthesized from 2-chlorobenzonitrile, which reacts with cyclopentylmagnesium bromide to give 1-(2-chlorobenzoyl)cyclopentane. The next step is bromination using bromine to the corresponding bromoketone, which upon reaction with an aqueous solution of methylamine forms the methylimino derivative. During this reaction a simultaneous hydrolysis of the tertiary bromine atom occurs. On further heating the reaction product in decalin, a ring expansion rearrangement occurs, causing formation of ketamine.

PHARMACOKINETICS
Routes of administration
Ketamine may be administered by a variety of routes as it is both water and lipid soluble. Intravenous, intramuscular, oral, rectal, subcutaneous, epidural and transnasal routes have all been used.
Bioavailability
| Route of administration | Bioavailability |
| Intravenous | 90% |
| Nasal | 50% |
| Oral | 20% |
| IM | 90% |
| Rectal | 25% |
| Epidural | 77% |
Metabolism and excretion
Ketamine is metabolized in the liver by the microsomal system, the major pathway is a N-demethylation which products the first metabolite, norketamine. This has a potency of around one-third that of ketamine. Norketamine is hydroxlyated to hydoxynorketamine, conjugated with glucuronate and then excreted renally with an elimination half-life of 2–3 h in adults.

• t1/2(distribution) = 11 - 16 minutes
• Vdss = 3 L/kg (very lipid soluble)
• clearance (Cl) = 12 - 17 ml/kg/min
• t1/2(elimination) = 2 - 3 hours

Plasma levels needed for hypnosis and amnesia during surgery are approximately 0.7 to 2.2 mcg/ml (perhaps up to 4.0 mcg/ml in children). Awakening occurs below 0.5 mcg/ml.
PHARMACODYNAMIC

Ketamine is a non-competitive NMDA receptors antagonist. This means that it doesn’t directly avoid the binding between glycine and the NR1 subunit of the receptor and between glutamate and the NR2 subunit.
Ketamine binds the receptor in a specific allosteric site localized inside the channel, preventing the molecular shape shifting and so avoiding its opening.
Thus the result is an inhibition of Na+ and Ca2+ flow into the cell and K+ out of the cell.
This molecular mechanism leads to the clinical effects of ketamine administration.
CLINICAL EFFECTS
Brain
The effect of Ketamine on the brain is substantially explained in the previous paragraph.
The inactivation of the NMDA receptors results in the dissociative anaesthesia, marked by catalepsy, amnesia and analgesia. The effects seem to take place mainly in the hippocampal formation and in the prefrontal cortex.
Cardiovascular system
Ketamine produces an increase in blood pressure, stroke volume and heart rate whilst maintaining systemic vascular resistance. These effects usually reach a maximum about 2 min after injection and settle over 15–20 min. There is a wide variation in individual response, and occasionally there can be a large rise in blood pressure, unrelated to a pre-operative history of hypertension. It is thought that these adrenergic responses are mediated centrally and the use of centrally depressant premedication such as benzodiazepines can blunt this effect.
These properties mean that ketamine is an ideal agent for the shocked patient but less appropriate for patients with severe ischaemic heart disease. Whilst ketamine has been shown to increase coronary blood flow, the benefit of this is probably negated by its effect on increased myocardial oxygen demand.
The effects of ketamine on the canine coronary circulation 2007.
Respiratory system
When ketamine is given slowly, respiration is usually well maintained. After rapid intravenous injection transient apnoea is occasionally seen, but this is easily managed with a brief period of bag-mask ventilation. These apnoeas are thought to be due to a reduced responsiveness to carbon dioxide with the high peak concentrations of ketamine seen after rapid injection.
Ketamine acts as a bronchodilator probably by two different mechanisms – firstly, via a central effect inducing catecholamine release, thereby stimulating β2 adrenergic receptors, resulting in bronchodilation, and secondly, via inhibition of vagal pathways to produce an anticholinergic effect acting directly on bronchial smooth muscle.
Ventilatory response to CO2 following intravenous ketamine in children 1989.
Skeletal muscle
Ketamine increases skeletal muscle tone. This is most noticeable after the initial intravenous bolus and gradually decreases. It may be improved by administration of benzodiazepines . It is rarely a problem intra-operatively although, in muscular young men, especially those requiring manipulation of fractures, relaxation with benzodiazepines or even muscle relaxants may be required.
Eyes
Induction with ketamine produces a small rise in intra-ocular pressure which is still sustained 15 min into anaesthesia. The putative mechanism for this rise is increased tone of the extra-ocular muscles coupled with increased blood flow due to the increased cardiac output and a rise in arterial pco2 seen with ketamine. Balanced anaesthesia with controlled ventilation helps to reduce these effects and in addition reduces the nystagmus otherwise commonly seen with ketamine anaesthesia.
Placenta
Ketamine crosses the placenta. Newborn infants after Caesarean section under ketamine anaesthesia will therefore be partially anaesthetised and should be cared for accordingly.
INDICATIONS
Anaesthesia
Ketamine produces dissociative anaesthesia (detached from surroundings). This is characterised by the patient often having their eyes open and making reflex movements during anaesthesia and surgery.
Sometimes these reflex movements can be troublesome, especially in fit, strong, young men. They may be decreased by a slight increase in dosage of ketamine, but further increases in dose are unlikely to improve the situation and may actually increase the reflex movements. Alternative strategies are to give further doses of benzodiazepines or analgesics (bearing in mind the increased risk of respiratory depression).
Frequent repeated sedation or anaesthesia with ketamine such as that often experienced by burns patients can lead to tolerance with increasingly large doses being required. This tolerance generally lasts for 3 days.
In recovery the patient may become agitated – this is due to hallucinations following ketamine anaesthesia. The reported frequency of these hallucinations varies widely from 5 to 30%. The incidence of hallucinations is lowest in children. Increased incidence is associated with female sex, large doses of ketamine and rapid intravenous boluses. Hallucinations can be reduced by premedication with benzodiazepines or, alternatively, promethazine, which has the added advantage of an anti-emetic effect.
One of the most effective methods to prevent emergence hallucinations is to allow the patient to recover undisturbed in a quiet area; however, in some tragic cases this has resulted in unsupervised recovery with fatal airway obstruction.
Since it suppresses breathing much less than most other available anaesthetics, ketamine is still used in human medicine as an anesthetic, however, due to the hallucinations, it is not typically the primary drug used. It is the the anaesthetic of choice when reliable ventilation equipment is not available. Ketamine tends to increase heart rate and blood pressure. Because of this, it is sometimes used in anesthesia for emergency surgery when the patient's fluid volume status is unknown; for example in the traffic accidents.
Use of ketamine in severe status asthmaticus in intensive care unit 2003
Analgesia
Ketamine is a potent analgesic and may be used as the sole analgesic agent intra-operatively. Balanced anaesthesia, with co-administration of opiates or tramadol intra-operatively, reduces the amount of ketamine required for maintenance of anaesthesia. This shortens the recovery time and reduces the incidence of some of the side-effects of ketamine but increases the risk of intra-operative respiratory depression.
In addition to its intra-operative analgesic effects, ketamine is increasingly used in both acute and chronic pain settings in the developed and developing world. It is thought that the mechanism for this may be related to the antagonistic effects of ketamine at the NMDA receptors, which are implicated in ‘wind-up’.
The glutamate interacts with the postsynaptic NMDA receptors, which aids the sensitization of the dorsal horn. This activation (by glutamate) enhances postsynaptic Nitric Oxide Synthase. Nitric Oxide is thought to migrate back to the presynaptic membrane to enhance the expression of the voltage-gated N-calcium channels resulting in a pain wind-up phenomenon. Ketamine and Nitric Oxide Synthase inhibitors can block pain wind-up. This abnormal central sensitization cycle results in increased pain (hyperalgesia) and pain responses from previously non-noxious stimuli evoke a pain response.

Ketamine as adjuvant analgesic to opioids: a quantitative and qualitative systematic review 2004
Use and efficacy of low-dose ketamine in the management of acute postoperative pain 1999
Complex regional pain syndrome
Ketamine is being used as an experimental and controversial treatment for Complex Regional Pain Syndrome. also known as Reflex Sympathetic Dystrophy (RSD). CRPS/RSD is a severe chronic pain condition characterized by sensory, autonomic, motor and dystrophic signs and symptoms. The pain in CRPS is continuous, it worsens over time, and it is usually disproportionate to the severity and duration of the inciting event. The hypothesis is that ketamine manipulates NMDA receptors which might reboot aberrant brain activity. There are two treatment modalities, the first consist of a low dose ketamine infusion of between 25–90 mg per day, over five days either in hospital or as an outpatient. This is called the awake technique.
The second treatment modality consists of putting the patient into a medically-induced coma and given an extremely high dosage of ketamine; typically between 600–900 mg. Usually the patient emerges from anesthesia completely free of pain and associated CRPS signs and symptoms.
Experimental antidepressant use
When treating patients suffering from complex regional pain syndrome (CRPS) with a low-dose (subanesthetic) ketamine infusion, it was observed that some patients made a significant recovery from associated depression. It was not possible to quantify to what degree depression recovery was secondary to the patient's recovery from CRPS.
The researchers apparently attribute the effect to ketamine being an NMDA receptor antagonist.
Indeed blocking the NMDA increases the activity of another receptor, AMPA, and this boost in AMPA activity is crucial for ketamine’s rapid antidepressant actions. NMDA and AMPA are receptors for the neurotransmitter glutamate. The glutamate system has been implicated in depression recently. This is a departure from previous thinking, which had focused on serotonin and norepinephrine. The glutamate system may represent a new avenue for treatment and research.

Faster-Acting Antidepressants Closer to Becoming a Reality 2007
Acute administration of ketamine at the higher dose, but not imipramine, increased BDNF protein. levels in the rat hippocampus. The increase of hippocampal BDNF protein levels induced by ketamine might be necessary to produce a rapid onset of antidepressant action in rats.

SIDE EFFECTS
Short term
- Increase in heart rate
- Slurred speech
- Confusion, disorientation
- Out-of-body experience
- Shifts in perception of reality
- Nausea
- Sedation
- Cardiovascular effects, including hypertension. and tachycardia
- Respiratory depression
- Hypersalivation
- Pleasant mental and/or body high
- Increase in energy
- Euphoria
- Sense of calm and serenity
- Spiritual experiences
- Enhanced sense of connection with the world (beings or objects)
- Distortion or loss of sensory perceptions (common)
- Open- and closed-eye visuals (common)
- Dissociation of mind from body
- Analgesia, numbness
- Ataxia
- Significant change in perception of time
- Double-vision
Long term
- Neurological effects:
Chronic use of ketamine may lead to cognitive impairments including memory problems.
The first large-scale, longitudinal study of ketamine users found that heavy ketamine users had impaired memory by several measures, including verbal, short-term memory and visual memory. However occasional (1-2 times per month) ketamine users and ex-ketamine users were not found to differ from controls in memory, attention and psychological well-being tests. This suggests that occasional use of ketamine does not lead to prolonged harm and that any damage that might occur may be reversible when ketamine use is stopped; however, depression worsened even in the abstinent user group over the period of the study (one year), along with dissociative symptoms still existing among infrequent users.
Ketamine: An Introduction for the Pain and Palliative Medicine Physician 2007.
Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study 2010.
- Urinary tract effects:
A study in Bristol reported in the British Medical Journal linked urinary tract disease with ketamine use. Symptoms reported by users included an increased need to urinate, passing blood in urine, leakage of urine and pain on urination. These symptoms may be associated with the scarification of the bladder lining, which leads to a shrunken bladder, erythema, and contact bleeding, and can then move to the ureters and damage the kidneys.
Urinary tract disease associated with chronic ketamine use 2008.
TOXICITY
OLNEY’S LESION.
In 1989, Olney et al. discovered that neuronal vacuolation and other cytotoxic changes ("lesions") occurred in brains of rats administered NMDA antagonists, including PCP., MK-801. and ketamine.
Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs 1989.
Examination of neurons in the posterior cingulate and retrosplenial cortices by electron micrograph revealed apparent lytic breakdown of mitochondria in the large vacuoles which had become apparent 2 hours after administration of an NMDA antagonist. The lowest doses of ketamine and tiletamine that produced neurotoxic changes visible by light microscope 4 hours post dose were 40 (mg/kg sc) and 10 (mg/kg sc), respectively. The potency of the drugs in producing these neurotoxic changes corresponded with their potency as NMDA antagonists: i.e. MK-801 > PCP > tiletamine > ketamine.
In medical settings, NMDA receptor antagonists are used as anesthetics, so GABA-A receptor agonists are used to effectively prevent any neurotoxicity caused by them.
NMDA receptor antagonist neurotoxicity and psychotomimetic activity 2003.
Hepato-toxicity in chronic pain management
Series of patients being treated with S(+)ketamine for relief of chronic pain. In three of the patients, abnormalities occur following repeat exposure to ketamine with the liver enzyme values returning below the upper reference limit of normal range on cessation of the drug.
Ketamine hepato-toxicity in chronic pain management 2011.
RECREATIONAL USE
Methods of use
Ketamine is sold in either powdered or liquid form. In its powdered form it can be insufflated (inhaled), injected, or taken orally (though this works as a laxative).
Ketamine is typically injected into the leg, the onset for IM is about one minute.
Oral use usually requires more material, but results in a longer trip. However, when administered orally, ketamine is rapidly metabolised to norketamine, which possesses sedating effects; this route of administration is unlikely to produce a dissociative state characteristic of ketamine unless very high doses are ingested.
Psychological effects
Ketamine is very short acting, its hallucinatory effects lasting sixty minutes when insufflated or injected and up to two hours when ingested, the total experience lasting no more than a couple of hours.
It produces a dissociative state, characterised by a sense of detachment from one's physical body and the external world which is known as depersonalization and derealization. At sufficiently high doses (e.g. 150 mg intramuscular), users may experience what is coined the K-hole , a state of dissociation whose effects are thought to mimic the phenomenology of schizophrenia. Users may experience worlds or dimensions that are ineffable, all the while being completely unaware of their individual identities or the external world. Users may feel as though their perceptions are located so deep inside the mind that the real world seems distant. Some users may not remember this part of the experience after regaining consciousness, in the same way that a person may forget a dream. Owing to the role of the NMDA receptor in long-term potentiation, this may be due to disturbances in memory formation. The "re-integration" process is slow, and the user gradually becomes aware of surroundings. At first, users may not remember their own names, or even know that they are human, or what that means. Movement is extremely difficult, and a user may not be aware that he or she has a body at all.
Essential Bibliography
Created by Fabio Baraldo