DEFINITION
Genetic-related coagulation disease (autosomical dominant ) with a high prevalence in the general population, characterized by a higher risk of venous thromboembolism.

HISTORY
Discovered in 1992 by Dahlback who reported a family in which 5 individuals spanning 3 generations had adult-onset thromboembolic disease, most often deep venous thrombosis of the legs, inherited in an autosomal dominant pattern. In this patient the aPTT didn’t increase after adding APC. Bertina in 1994 found that it was caused by a mutation in factor V gene; mutated protein had been called factor V Leiden. Greengard in the same year understood that the mutation wasn’t enough to clinical manifestation but it needed risk factors. In a population-based cohort study of 9,253 Danish adults, Juul et al. (2004) found that heterozygotes and homozygotes for factor V Leiden had , respectively ,6 and 60 times higher risk for venous thromboembolism than noncarriers. Heresbach et al. (1997) added small bowel infarctions to the many thrombotic states in which the factor V Leiden mutation plays a role. Mahmoud et al. (1997) reported the incidence of the factor V Leiden mutation in Budd-Chiari syndrome and portal vein thrombosis.

Normal allelic variants . Haplotype analysis of the factor V gene strongly suggests that the mutation at nucleotide 1691 was a single event that occurred 20,000-30,000 years ago, after the evolutionary separation of whites from Asians and Africans. Some investigators speculate that the mild hypercoagulable state conferred by the mutation could have had a beneficial effect in reducing mortality from bleeding associated with childbirth or trauma in pre-modern times. Factor V Leiden heterozygotes undergoing elective cardiac surgery had significantly less blood loss and a lower risk of requiring a blood transfusion than individuals with a normal factor V genotype . Another study suggested that the mutation is associated with a fivefold lower risk for spontaneous intracranial hemorrhage, consistent with the proposed protective effect . A study of women who had successful in vitro fertilization suggested that factor V Leiden enhances embryo implantation, thereby favoring the early survival of heterozygotes . Analysis of a large randomized trial of individuals with severe sepsis showed that factor V Leiden heterozygotes had a threefold greater probability of survival, confirming animal models of sepsis that suggest a similar survival benefit . Although each of these hypothesized beneficial effects could account for the persistence of the mutation, a survival advantage remains to be confirmed.
EPIDEMIOLOGY
The prevalence of heterozygous in Italy is 2-5%, in Europe 1-4% with high geographical differences (14% in Greece, and in absence in populations of non-Caucasian.) .The homozygous patients are about 1:6000-7000 in Italy. General population screened for FV Leiden showed low absolute incidence of VTE (Venous ThromboEmbolism) of approximately two events per 1000 persons per year in heterozygotes while homozygotes had an absolute incidence of 15 VTE events/1000 persons/year.
PATHOGENESIS AND GENETICS
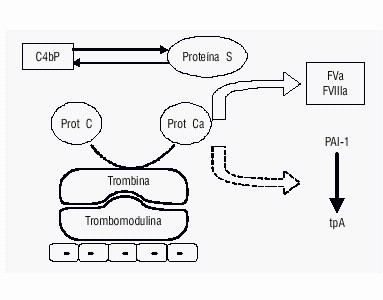
The PC is synthesized in liver. It’s a vitamin k-dependent zimogen with 6 hours half-life. It’s encoded by a gene sited on CR2q14. It has one domain for interaction with phospholipids and Ca 2+ and a serin-protease domain activated by thrombin which exposes the PC active site .This activation is slow and intervenes in the presence of trombomodulin. PC is an inhibitor of coagulation factors Va and VIIIa; it carry out its inhibitory action with the aid of protein S in presence of Ca2+ and phospholipids.
One of the ligation site of factor V with active PC is located in arginine aminoacid , in 506 position. The mutation is known as VR506Q, and consist in the sostitution of a guanine in an adenine in 1691 position in CR1q24 , with consequent sostitution of an arginine with a glutamine in 506 position. Accordingly, factor V is no longer sensitive to the inactivation made by activated PC and an increase of production of thrombin with a pro-coagulant effect, which predisposes to thrombosis, is favored. The factor V Leiden maintains a normal coagulant activity when activated by thrombin and factor Xa but the binding affinity with PC and PS is reduced and the speed of inactivation is 10-20 times slower.. have been recently identified two other mutations of the factor V associated with thrombophilia .The first, H1299R, consists in the replacement of an adenine with a guanine at nucleotide position 4070 in exon 13. (The term "factor V Leiden" refers to the specific G-to-A substitution at nucleotide 1691 in the gene for factor V that predicts a single amino-acid replacement (R506Q) at one of three APC cleavage sites in the factor Va molecule). Such substitution at the protein level implies the insertion of an arginine in place of a histidine at amino acid position 1299. Affected individuals have two chance of transmitting the predisposition to their children, regardless of sex. The second, Y1702C, consists in the replacement of an adenine with a guanine at position 5279 of the gene, and at the protein level involves the replacement of a
tyrosine with a cysteine residue in position 1702 of the factor V coagulation. Genetic evidence, structural and functional support the association of the Factor V mutation Y1702C with an increased risk of 3-4 times to develop thrombotic events.
SYMPTOMS
Symptoms are to be referred to thrombotic events such as the increased risk of DVT in the legs or in unusual locations such as the portal vein, retinal and placental veins with risk of abortion IUGR preeclampsia placental abrutio, even in young patients. The clinical expression of factor V Leiden thrombophilia is influenced by: (1) the number of factor V Leiden alleles (heterozygotes have a slightly increased risk for venous thrombosis; homozygotes have a much greater thrombotic risk); (2) coexisting genetic thrombophilic disorders, which have a supra-additive effect on overall thrombotic risk; (3) acquired thrombophilic disorders: antiphospholipid antibodies, hyperhomocysteinemia, high factor VIII levels, malignancy; (4) circumstantial risk factors: travel, central venous catheters, pregnancy, oral contraceptive use, hormone replacement therapy (HRT), selective estrogen receptor modulators (SERMs), organ transplantation, advancing age, and surgery.

DIAGNOSIS
The diagnosis of factor V Leiden thrombophilia is made either using a coagulation screening test or by DNA analysis of the F5 gene, which encodes the factor V protein.
Factor V Leiden testing may be considered in the following individuals:
- Women with unexplained fetal loss after ten weeks’ gestation
- Selected women with unexplained severe preeclampsia/ ”HELLP” (hemolysis, elevated liver enzymes and low platelets), placental abruption, or a fetus with severe intrauterine growth restriction
- A first VTE related to the use of tamoxifen or other selective estrogen receptor modulators (SERMs)
- Female smokers younger than age 50 years with a myocardial infarction or stroke
- Individuals older than age 50 years with a first provoked VTE in the absence of malignancy or an intravascular device
- Asymptomatic adult family members of probands with a known factor V Leiden mutation, especially those with a strong family history of VTE at a young age
- Neonates and children with non-catheter related idiopathic VTE or stroke
The APC resistance assay involves performing an aPTT on the individual’s plasma in the presence and absence of a standardized amount of exogenous APC; the two results are expressed as a ratio (aPTT + APC / aPTT - APC). This assay is based on the principle that when added to normal plasma, APC inactivates factors Va and VIIIa, which slows coagulation and prolongs the aPTT. The APC-resistant phenotype is characterized by a minimal prolongation of the aPTT in response to APC and a correspondingly low ratio. The addition of the factor V-deficient plasma corrects for deficiencies of all other coagulation proteins, neutralizes therapeutic concentrations of heparin, and also eliminates the effect of some lupus inhibitors In the modified (“second generation”) assay currently available, the individual's plasma is first diluted (1:4) in factor V-deficient plasma that contains polybrene, a heparin neutralizer. The assay can be used for individuals receiving warfarin or heparin anticoagulation and for many individuals with lupus inhibitors, as well as in the setting of acute thrombosis, pregnancy, or inflammation. This test has a sensitivity and specificity for factor V Leiden approaching 100%.
Although the modified APC resistance assay is highly sensitive, DNA-based testing is recommended in individuals with the following:
Strong lupus inhibitors and a markedly prolonged baseline aPTT
Very low APC resistance assay values, in order to differentiate heterozygotes, homozygotes, and "pseudohomozygotes" who are heterozygous for both factor V Leiden and a second mutation causing a factor V deficiency
Borderline APC resistance assay values
THERAPY
Thrombosis
The management of individuals with factor V Leiden thrombophilia depends on the clinical circumstances.
The first acute thrombosis should be treated according to standard guidelines with a course of low molecular-weight heparin or intravenous unfractionated heparin. Oral administration of warfarin is started concurrently with low molecular-weight heparin (except during pregnancy) and monitored with the international normalized ratio (INR). A target international normalized ratio (INR) of 2.5 (therapeutic range 2.0-3.0) provides effective anticoagulation, even in individuals with homozygous factor V Leiden .Low molecular-weight heparin and warfarin therapy should be overlapped for at least five days, and until the INR has been within the therapeutic range. Low molecular-weight heparin and warfarin are both safe in breast-feeding women.
The duration of oral anticoagulation therapy should be tailored to the individual, based on an assessment of the risks for VTE recurrence and anticoagulant-related bleeding. Approximately 30% of individuals with an incident VTE develop recurrent thrombosis within the subsequent ten years. Individuals with a spontaneous thrombosis and no identifiable provoking factors or persistent risk factors require a longer course of anticoagulation. In contrast, individuals with transient (reversible) risk factors such as surgery require a shorter course of therapy
The risk for VTE recurrence is higher in persons with proximal than with distal DVT and in those with one or more prior episodes of VTE. Other risk factors for recurrent VTE include male sex and a negative D-dimer level one month after discontinuation of warfarin.
Long-term oral anticoagulation should be considered in individuals homozygous for the factor V Leiden mutation or with multiple thrombophilic disorders.
Individuals receiving long-term therapy should be reevaluated at periodic intervals to confirm that the benefits of anticoagulation outweigh the bleeding risks.
Unfractionated and low molecular-weight heparin, fondaparinux (a pentasaccharide), and warfarin are the primary antithrombotic agents used for the acute and long-term treatment of arterial and venous thromboembolism. Several direct thrombin inhibitors (lepirudin dabigatran) are approved for use in certain circumstances.
Graduated compression stockings should be worn for at least two years following an acute DVT.
BIBLIOGRAFY
OMIM
Wikipedia
GeneReviews
PubMed
Matteo Dosio Paolo Arnoffi Gabriele Torta