Background
Impaired glucose tolerance, impaired fasting glucose and type 2 diabetes are conditions characterised by varying levels of insulin resistance causing hyperglycaemia on the background of an insulin secretion defect. Patients with IGT and/or IFG do not have as severe insulin resistance and hyperglycaemia as those with T2DM
and they are often regarded as having pre-diabetes. Rising levels of obesity, insufficient physical activity and high levels of uninterrupted sedentary time are independent risk factors for insulin resistance/diabetes. (Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diabetes Metab Res Rev 2009)..
Fasting blood glucose homeostasis.
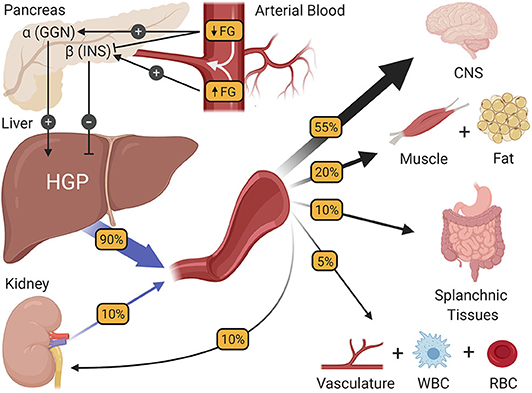
- The regulation of fasting blood glucose homeostasis. Subtle changes in fasting glucose levels (FG) entering the pancreas regulate the release of the islet hormones insulin (INS) and glucagon (GGN). In turn, these hormones control the rate of hepatic glucose production (HGP), making HGP equal to the rate at which all other tissues of the body utilize glucose, thereby preserving fasting glucose levels at a steady state. CNS, central nervous system; WBC, white blood cells; RBC, red blood cells.
Exercise-Induced Improvements to Whole Body Glucose Metabolism in Type 2 Diabetes: The Essential Role of the Liver, 2020
Role of exercise in the prevention and treatment of T2DM and pre-diabetes.
Exercise training, is a recognised, although relatively underutilised strategy that is central to the prevention, care and management of T2DM and pre-diabetes. (Standards of medical care in diabetes. Diabetes Care 2008).
The U.S. Diabetes Prevention Program reported a 58% reduction in the incidence of T2DM from a four-year lifestyle intervention that prescribed 150 min/week of moderate activity exercise and dietary change program designed to induce a 7% weight loss. Importantly, the patients actually completed on average approximately 300 min/week at this intensity. (Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002).
A similar risk reduction of 58% was reported in the Finish Diabetes Prevention Study that prescribed 210 min/week of moderate to strenuous intensity exercise (including resistance training) and dietary intervention to reduce fat and increase fibre intake, with patients completing an average of 204 min/week of exercise. (Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001)
Biochemistry
Skeletal muscle is the primary site of glucose disposal under insulin-stimulated conditions. In response to insulin binding, insulin receptors on skeletal muscle cells undergo autophosphorylation on tyrosine residues, leading to activation of the receptor tyrosine kinase and subsequent tyrosine phosphorylation of insulin receptor substrate-1 (IRS- 1). (Exercise Training Increases Glycogen Synthase Activity and GLUT4 Expression But Not Insulin Signaling in Overweight Nondiabetic and Type 2 Diabetic Subjects. Metabolism Clinical And Experimental, 2004) IRS-1 in turn associates with the regulatory subunit of phospatidylinositol 3 (PI 3)-kinase, activating the P110 catalytic subunit, (The IRS-signalling system: A network of docking proteins that mediate insulin action. Mol Cell Biochem 182) which is necessary for mediating insulin’s metabolic effects, including GLUT4 translocation, glucose disposal, and increasing the activity of glycogen synthase and hexokinase. (Regulation of hexokinase II and glycogen synthase mRNA, protein, and activity in human muscle. Am J Physiol, 1995)
Insulin resistance in skeletal muscle is characterized by decreased insulin-stimulated glucose disposal, as well as decreased hexokinase and glycogen synthase activity. On a molecular level, it has been found insulin’s activation of the IRS-1 PI 3-kinase signaling pathway to be reduced in skeletal muscle of rodents and humans with insulin resistance and type 2 diabetes. (Altered expression levels and impaired steps in the pathway to phosphatidylinositol 3-kinase activation via insulin receptor substrates 1 and 2 in Zucker fatty rats. Diabetes, 1998).
GLUT4
Douen et al. (Exercise induces recruitment of the insulin-responsive glucose transporter. Evidence for distinct intracellular insulin and exercise-recruitable transporter pools in skeletal muscle. J. Biol. Chem. 1990.) developed some studies with the purpose of explaining the two different mechanisms that stimulate glucose transport due to the action of insulin and muscle contraction. One hypothesis is that there are two separate pools of GLUT4 in the skeletal muscle. GLUT4 from one pool is thus translocated by the action of insulin but not of contraction, whereas GLUT4 from another pool is translocated by contraction stimulation, but not by insulin.
Ploug et al. (Analysis of GLUT4 distribution in whole skeletal muscle fibers, identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell. Biol., 1998.) showed GLUT4 to be in two kinds of depots: large elements (including multivescicular endosomes associated with the Golgi complex), which include ≈23% of total GLUT4, surround the nuclei and form long rows between the nuclei and in the core of the fibers, and small elements (tubulovescicular structures), which comprise ≈77% of total GLUT4, are found throughout the fibers. The small GLUT4 depots are seen throughout the fibers. They are not associated with the Golgi complex but ≈52% appear to be associated with transferrin receptor (TfR) endosomes. The subcellular fraction experiments demonstrated that insulin stimulation results in translocation of GLUT4 with a minimal effect, if any effect at all, on TfR. In contrast, contractions result in a substantial translocation of both GLUT4 and TfR. This indicates that insulin recruits GLUT4 positive/TfR-negative vesicles, whereas contraction recruits GLUT4-positive/TfR-positive vesicles. These data are in agreement with the notion that insulin and contractions recruit GLUT4 from different intracellular pools (Table I).

Table I:_Fibers_ from each of the four experimental groups were labeled with anti-GLUT4 and processed for immunogold electron microscopy. Silver-enhanced gold grains were counted from longitudinal sections. All results are given as means ± SEM. Numbers in parentheses represent the number of independent experiments counted, followed for rows 1 and 2 by the total length of membrane counted (in μm), and for rows 2 and 3 by the number of triads counted.
* Number of grains/10 μm of plasma membrane. The background labeling in the absence of primary antibody was 2.8 ± 0.2 grains/10 μm and has been subtracted from the presented data.
‡ Number of grains/10 μm of longitudinally sectioned junctional T tubule membrane. The background labeling in the absence of primary antibody was 1.2 ± 0.7 grains/10 μm and has been subtracted from the presented data
It is also known that some SNARE proteins (VAMP2, Syntaxin4, Snap25, Munc18) regulate the docking and fusion of GLUT4 vescicles with the plasma membrane. (Effect of insulin and contraction up on glucose transport in skeletal muscle. Progress in Biophysics & Molecular Biology 2004). Exercise induces increases VAMP2 in the plasma membrane (Exercise-induced increases in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am. J. Physiol. 1996.)

Insulin Sensitivity
The improvements in insulin sensitivity after exercise training may be related to changes in expression and/or activity of proteins involved in insulin signal transduction in skeletal muscle. Zierath (Exercise effects of muscle insulin signaling and action: invited review: exercise training-induced changes in insulin signaling in skeletal muscle. J. Appl Physiol., 2002.) recently reviewed the effects of exercise on insulin signalling in skeletal muscle (Fig. 1). The author reports that exercise training appears to increase insulin sensitivity by enhanced post-receptor insulin signalling at the level of IRS proteins and PI3-kinase. Insulin-stimulated PI3-kinase activity is impaired in skeletal muscle from Type 2 diabetic and obese insulin-resistant subjects. Enhanced phosphotyrosine-associated PI3-kinase activity (Insulin signaling after exercise in insulin receptor substrate-2 deficient mice. Diabetes, 2002.) in the hours after exercise may partly contribute to the persistent increase in glucose uptake after exercise. Regular exercise training enhances insulin-stimulated PI3-kinase activity in skeletal muscle (Effect of short-term exercise training on insulin-stimulated PI3-kinase activity in human skeletal muscle. Am J Physiol Endocrinol Metab, 1999.). Because PI3-kinase is an important regulatory step for glucose transport, increased signal transduction at this key step after exercise training may contribute to the exercise-associated increase in insulin action in skeletal muscle. Increased mRNA levels of the p85 -subunit of PI3-kinase have been noted in rodents and humans engaged in acute (Differential effects of exercise on insulin-signaling gene expression in human skeletal muscle. J Appl Physiol, 2001.) or long-term (Exercise effects of muscle insulin signaling and action: invited review: exercise training-induced changes in insulin signaling in skeletal muscle. J. Appl Physiol., 2002.) exercise training; however, the physiological significance of this is unknown.

AMPK and Ca2+
Two elements have been proposed as contributors to the contraction-induced glucose uptake. The first one is the metabolite AMP, produced as a result of ATP degradation during muscle contraction. The second one is Ca2+ release from sarcoplasmic reticulum stores upon depolarization of membranes at the transverse tubules. AMP induces AMPK, while Ca2+ is involved in activation of some PKC isoforms, but also binds to several proteins associated with calmodulin. Calmodulin inhibitors render controversial results on the involvement of Ca2+/calmodulin in contraction-induced glucose uptake (Signalling to glucose transport in skeletal muscle during exercise. Acta Physiol Scand 2002.).
In human skeletal muscle, AMPK activity is dependent not only on the energetic status of the cell, but also on the ‘fuel’ status, such that in situations of low muscle glycogen content, AMPK activity is elevated. Regulation of 5¢AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab, 2003.).
Two proteins are essential for the regulation of GLUT-4 expression at a transcriptional level: myocyte enhancer factor 2 (MEF2) and GLUT-4 enhancer factor (GEF). Mutations in the DNA binding regions for either of these proteins results in ablation of transgene GLUT-4 expression. (Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 2000). These results prompted a study in 2005 which showed that AMPK directly phosphorylates GEF, but it doesn’t seem to directly activate MEF2.
Exercise guidelines
The American College of Sports Medicine and American Diabetes Association suggest minimum exercise prescription recommendations for patients with T2DM or pre-diabetes. The recommendations are shown in Table 1. (Exercise prescription for patients with type 2 diabetes and pre-diabetes: A position statement from Exercise and Sport Science Australia. Journal of Science and Medicine in Sport, 2012)
 Table 1:
Table 1:
The VO2 reserve is the difference between resting and peakVO2, and, as it describes the O2 used during exercise in addition to basal consumption, is considered a direct measure of the exercise load or energy expenditure.
HRmax: 220-age
HRR: HRmax-HRrest
RPE: The RPE Scale is a common method for determining exercise intensity levels. The scale of perceived exertion is how hard you feel your body is working, and therefore is a subjective measurement.
The total amount of exercise should consist of a combination of aerobic and resistance training. It is recommended that resistance training (2–4 sets of 8–10 repetitions) should make up two or more sessions each week. Aerobic and resistance training can be combined in the one session. Exercise should be performed on at least 3 days each week with no more than two consecutive days without training. The exercise recommendation can be achieved with a combination of moderate and vigorous intensity exercise, if desired and clinically feasible. When using a combination, we suggest that the vigorous intensity exercise can be multiplied by 1.7 to allow this to be added to the moderate intensity time. For example, in one week a person could exercise on four days for 40 min at a moderate intensity and on another day for 30 min at a vigorous intensity. The 1.7 multiplication factor was derived from recommendations that 150 min of moderate intensity is equivalent to 90 min of vigorous physical activity (a ratio of 1:1.7). (Exercise training for type 2 diabetes mellitus: impact on cardiovascular risk: a scientific statement from the American heart association. Circulation, 2009).
Beneficial effects have been shown with both aerobic, resistance (Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med, 2007; Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look ahead trial. Diabetes Care 2007) or a combination of both modes of training, in which case they have shown to be synergistic and may yield greater results than each mode of exercise alone. (Effects of aerobic resistance training on hemoglobin a1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 2010.). These improvements in glycaemic control commonly result in reductions in T2DM medications. (Effects of a short-term circuit weight training program on glycaemic control in niddm. Diabetes Res Clin Pract 1998.)
The American Diabetes Association (ADA) recommends that carbohydrate ingestion is needed for pre-exercise blood glucose levels of <100 mg/dL (<5.5 mM) ; however, this recommendation would apply only to people with type 1 or type 2 DM who are taking supplemental insulin injections or using certain sulfonylureas. (Glycemic response during exercise after administration of insulin lispro compared with that after administration of regular human insulin. Diabetes Res Clin Pract., 2002.). For people whose disease is controlled with diet or oral antidiabetic medications alone, extra carbohydrates are generally not required during exercise lasting <1 hour. Clinicians recommend that people with type 2 DM who are treated with insulin ingest at least 15 g of carbohydrate before exercise for a starting blood glucose level of <100 mg/dL; the exact quantity depends on other variables such as when the injected insulin peaks and the duration of the activity. In all cases, intense, short periods of exercise would require a lesser carbohydrate intake, if any, due to a greater release of counterregulatory hormones. (Combined infusion of epinephrine and norepinephrine during moderate exercise reproduces the glucoregulatory response of intense exercise. Diabetes. 2003).
Individuals with DM should not let more than 2 days lapse between bouts of exercise and that they exercise at least 3 nonconsecutive days per week. (Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004).
Ideally the total amount of exercise should consist of some aerobic and some resistance training, however, if only one modality can be performed due to comorbidities, behavioural considerations or other constraints, then either modality alone has been shown to be effective. The risks associated with exercise are considered less than those of inactivity, even in older adults with multiple chronic diseases. Therefore, exercise training should be an essential component of any treatment plan for all patients at risk of or with T2DM. Due to the potential risks and likelihood of the presence of comorbidities, programs should be designed and delivered by qualified personnel who are trained and experienced to deal with the likely additional considerations. (Exercise prescription for patients with type 2 diabetes and pre-diabetes: A position statement from Exercise and Sport Science Australia. Journal of Science and Medicine in Sport 2012).