POTENTIAL ROLE OF BEER IN CANCER PREVENTION
Introduction. 
Beer is one of the most commonly consumed alcoholic beverages. It is rich in nutrient components including carbohydrates, aminoacids, minerals, vitamins, and phenolic compounds.
According to the Beer Purity Law from 1516, traditional German beer is made only from barley, hop, and water, with the addition of yeast. Various parameters during brewing, the variety of barley and the malting process, temperature and pH during mashing, sparging, boiling, the variety of hops added during wort-boiling, and yeast fermentation, influence the type and quality of beer.
Like that of many other traditional beverages, beer comsumption was originally emblematically associated with enhanced health.
However the identification of increasing numbers of pharmacologically active compounds from natural products and examination of their molecular mechanisms is opening new perspectives also for this beverage, particularly in preventive medicine.
Beer constituents as potential cancer chemopreventive agents. Gerhauser, C. (2005) Eur.J.Cancer 41, 1941–1954
In fact over 60% of the the current anticancer drugs have their origin in one way or another from natural sources. Nature continues to be the most prolific source of biologically active and
diverse chemotypes, and it is becoming increasingly evident that associated microbes may
often be the source of biologically active compounds originally isolated from host macroorganisms. While relatively few of the actual isolated compounds advance to become clinically effective drugs in their own right, these unique molecules may serve as models for the preparation of more efficacious synthetic analogues, underlying the essential role played by natural products in the discovery and development of effective anticancer agents.
Nature: a vital source of leads for anticancer drug development From the issue entitled Natural Products in Cancer Therapy (PSE meeting Naples, 23 -26 September 2008) / Guest Edited by V. Lanzotti
Studies of several compounds of plant origin, especially the flavonoids, suggest that these molecules can harbor cancer chemopreventive.
Flavonoids are a group of constituents of food and drinks commonly consumed by humans, including compounds of different chemical classes such as flavones (7,8-benzoflavone), flavonols (quercetin), flavanol (cathechin), flavanones (naringenin), isoflavones (genistein), and chalcones (xanthohumol). Xanthohumol (XN) has been isolated from hop “cones”, the female inflorescences of the hop plant (Humulus lupulus L.) that are largely used in the brewing industry as a preservative and flavoring agent to add bitterness and aroma to beer.

Chemically, XN belongs to the prenylated chalcones (open C-ring flavonoids) class and represents 82–89% of the total prenylated flavonoid identified in various European hop varieties. In beer (the major dietary source of prenylated flavonoid) it has been found at concentrations up to 0.96 mg/l (1.95 µM). The biological effects of the chalcones depend on their chemical structure, and substitutions on the structure have a profound influence on the anticarcinogenic effects of the compounds.

Cancer chemopreventive activity Xanthohumol, a natural product derived from hop. Gerhauser, C., Alt, A., Heiss, E., Gamal-Eldeen, A., Klimo, K. Knauft, J.Neumann, Scherf, H. R., Frank, N. Bartsch, H. et al. (2002)Mol. Cancer Ther. 1, 959–969
Among many pathways on which XN seems to have effects, in last ten years it was evaluated, with increasing interest, the action that this substance has on the NF-kB pathway, strictly implicated in the activation of genes promoting tumoral growth.
NF-kB influence numerous gene products involved in tumor and endothelial invasion, including matrix metalloproteinases (MMPs), urokinase type plasminogen activator (uPA), interleukin-8 (IL-8), and other chemokines . NF-κB activation also controls the expression of a number of chemokines and angiogenic growth factors; various adhesion molecules, including ICAM-1, VCAM-1, and ELAM-1, and even iNOS. Furthermore, NF- kb protects cells from the induction of programmed cell death by proapoptotic stimuli such as tumor necrosis factor (TNF).
Nuclear factor-κB: the enemy within. Aggarwal, B. B. (2004) Cell 6, 203–208
In a resting state, inhibitory proteins, known as the IκBs, repress resident NF-κB activity by binding it to prevent nuclear uptake.
Inactive NF-κB consists of a heterotrimer composed of the p50 and p65 subunits together with the IκB complex. Proinflammatory stimulation like TNF leads to rapid phosphorylation of IκBa (induced by the protein kinase IKK). The phosphorylation of IκBa leads to its ubiquitination and rapid degradation that consequently frees the p50-p65 NF-κB heterodimer to translocate to the nucleus and activate the trascription of his dependent genes.
NF-kB
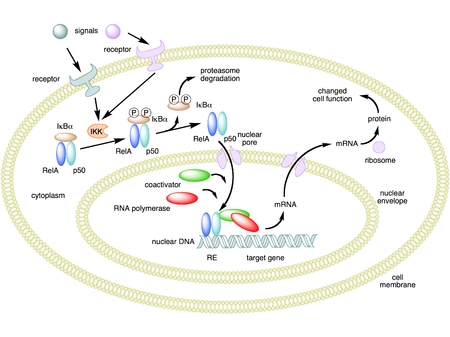
XN inhibits NF-κB translocation and activation
Tumor invasion is influenced by numerous gene products that are regulated by NF-κB. This
nuclear factor also regulates aspects of inflammation and the expression of a number of
chemokines and angiogenic growth factors.
In 2005 was published an article (" mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol;NF-kB and Akt as targets ") in which is established that XN could affect the NF-κB pathway. In that article this theory was confirmed by many analysis;
Immunofluorescent staining indicated that NF-κB was largely localized in the cytoplasm of unstimulated HUVEC (Human umbilical vein endothelial cells) and that XN treatment had little effect on this localization.
Treatment of endothelial cells for 15 min with 10 ng/ml TNF-α resulted in translocation of NF-κB to the nucleus; pretreatment with 10 µM XN completely inhibited this translocation, with NF-κB remaining in the cytoplasm.
In keeping with this, ELISA assays for activated NF-κB demonstrated a significant reduction in the amount of active NF-κB upon XN treatment in both untreated and TNF-α-stimulated cells.
Western blot analysis indicated that XN also significantly repressed the levels of phosphorylated IκBα present in HUVEC after stimulation with TNF-α, indicating an inhibitory activity by XN toward the protein kinase IKK, which phosphorylates IκBα.
Mechanisms of the antiangiogenic activity by the hop flavonoid xanthohumol: NF-kB and Akt as targets. Adriana Albini,Raffaella Dell’Eva,Roberta Vené,Nicoletta Ferrari,Donald R. Buhler,Douglas M. Noonan and Gianfranco Fassina. The FASEB Journal express. Published online December 30, 2005
These data suggest that XN is able to interfere with the signaling pathways leading to NF-κB activation but, despite these experimental evidences, the molecular mechanisms underlying the process is not yet fully understood.
Another study published in 2009 (“ Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB–regulated gene products and potentiation of apoptosis in leukemia cells ”) helped to define with more specificity the molecular interaction responsible of the NF-Kb inhibition.
It was found that XN inhibits NF-κB expression induced by inflammatory stimuli, such as TNF, IL-1β, pro-oxidants, carcinogens and tumor promoters. The inhibition of NF-κB expression induced by these agents suggests that XN must act at a step that is common to all of these many agents.
XN inhibits TNF-induced IκBα phosphorylation and degradation, thus leading to suppression of nuclear translocation of NF-Kb through the inhibition of TNF-induced IKK activation.The study evidence indicates that XN affects IKK through direct interaction;
The presence of a reducing agent reverses the effect of XN on IKK, suggesting a role for cysteine residues, couple of amino acid structures present on IKK that form the covalent bond called disulfide bridge (the disulfide bridges between the cysteine residues stabilize the structure of many proteins).
Furthermore, mutation of IKK-β from cysteine to alanine at position 179 abolished the effect of XN on inhibition of IKK activity. How XN modifies cysteine 179 of IKK is not fully understood. All these results suggest that XN is interacting with cysteine residue of IKK directly. XN contains an electrophilic carbonyl group and one Michael acceptor, which could interact directly with the sulfhydryl group of cysteine residue, thus leading to an adduct formation.
p<>. Besides its interaction with IKK, it was found that XN also inhibited NF-κB directly, through its interaction with the p65 subunit of NF-κB. Specifically, it was observed that XN inhibits the binding of p65 to DNA both in vivo and in vitro. This inhibition of binding by XN again could be reversed by reducing agents, suggesting a role for cysteine residue, present also on NF-kB.
This is a general mechanism for polyphenols, which possess unsaturated carbonyl structures; These functional groups are known to react with nucleophiles, especially with cysteine sulfhydryl groups, in a Michael-type addition.
Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB–regulated gene products and potentiation of apoptosis in leukemia cells
Kuzhuvelil B. Harikumar, Ajaikumar B. Kunnumakkara, Kwang S. Ahn, Preetha Anand, Sunil Krishnan, Sushovan Guha and Bharat B.Aggarwal
Conclusions
XN could be a potent orally available anti-tumoral and chemopreventive agent whose mechanism targets endothelial cell migration and invasion and to a lesser extent proliferation. The mechanism of action of this molecule is not completely known but seems related to a steric interaction with more than one protein involved in signalling of NF-kB pathway.
The levels of XN in beer indicate however that a daily intake of numerous liters might be necessary to attain significant chemoprevention effects. Therefore, enrichment of XN in the beer extraction in brewing and/or the isolation of XN administered as a dietary supplement is already investigating in new trials.
Some German laboratories are trying to create new types of beer with a higher content of XN, thereby reducing the amount of drink needed to obtain an antitumor effect.
Daniele Amparore
Andrea Bonani