DESCRIPTION
Donepezil is reversible inhibitor of acetylcholinesterase, centrally active, used for treatment of Alzheimer's disease where it is used to increase cortical acetylcholine.
CLASSIFICATION
NAME: Donepezil
TYPE/GROUP: a piperidine (an heterocyclic amine (CH 2) 5 NH) derivative
 Donepezil
Donepezil
 Piperidina
Piperidina
CATEGORY:
- Parasympathomimetics
- Cholinesterase Inhibitors
- Nootropic Agents
MOLECULAR FORMULA: C 24-H 29-NO 3
MOLECULAR WEIGHT: 379.49 g/mol
IUPAC NAME: 2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimethoxy-2,3-dihydro-1H-inden-1-one
(Source: PubChem. Keyword: Donepezil; Drug Bank)
INDICATION
Donepezil is primarily used in the treatment of Alzheimer’s diseas.Donepezil has been tested in other cognitive disorders including Lewy body dementia and Vascular dementia, but it is not currently approved for these indications. It has also been studied in patients with Mild Cognitive Impairment, schizophrenia, attention deficit disorder, post-coronary bypass cognitive impairment, cognitive impairment associated with multiple sclerosis, and Down syndrome.
Donepezil’s effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. (Source: PubChem. Keyword: Donepezil; Drug Bank)
PHARMACOKINETICS
Donepezil is well absorbed with a relative oral bioavailability of 100% and reaches peak plasma concentrations in 3 to 4 hours. Pharmacokinetics are linear over a dose range of 1 to 10 mg given once daily. Neither food nor time of administration (morning vs. evening dose) influences the rate or extent of absorption of donepezil hydrochloride tablets.
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=759]6
The mean apparent plasma clearance (Cl/F) is 0.13 L/hr/kg; following multiple dose administration, donepezil accumulates in plasma by 4 to 7 fold and steady state is reached within 15 days. The steady state volume of distribution is 12 L/kg.
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=7596]
In patients with stable alcoholic cirrhosis, the clearance of donepezil hydrochloride was decreased by 20% relative to healthy subjects.
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=7596]
In patients with moderate to severe renal impairment (ClCr < 18 mL/min/1.73 sq m) the clearance of donepezil hydrochloride did not differ from healthy subjects.
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=7596] PEER REVIEWED
Donepezil is approximately 96% bound to human plasma proteins, mainly to albumins (about 75%) and alpha1-acid glycoprotein (about 21%) over the concentration range of 2 to 1000 ng/mL.
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=7596]
It is not known whether donepezil is excreted in human breast milk and it is not known whether donepezil hydrochloride and/or its metabolites can be removed by dialysis (hemodialysis, peritoneal dialysis, or hemofiltration).
BIOLOGICAL HALF-LIFE: the elimination half life of donepezil is about 70 hours.
ROUTE OF ELIMINATION: Donepezil is both excreted in the urine intact and extensively metabolized to four major metabolites, two of which are known to be active, and a number of minor metabolites, not all of which have been identified.
METABOLISM: Donepezil is metabolized by CYP 450 isoenzymes 2D6 and 3A4 in the liver and also undergoes glucuronidation. The main metabolite, 6-O-desmethyl donepezil, has been reported to inhibit AChE to the same extent as donepezil in vitro. (Source: Drug Bank)
MOLECULAR MECHANISM OF ACTION:
The precise mechanism(s) of action of donepezil in patients with dementia of the Alzheimer's type (Alzheimer's disease) has not been fully elucidated. The drug is an anticholinesterase agent that binds reversibly with and inactivates cholinesterases (eg, acetylcholinesterase), thus inhibiting hydrolysis of acetylcholine. As a result, the concentration of acetylcholine increases at cholinergic synapses. In vitro data and data in animals indicate that the anticholinesterase activity of donepezil is relatively specific for acetylcholinesterase in the brain compared with butyrylcholinesterase inhibition in peripheral tissues.
[American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1293]
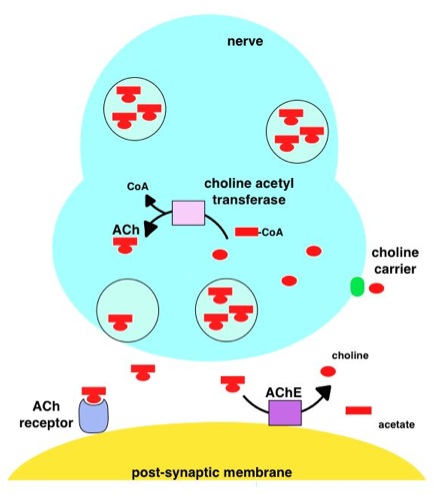
Acetylcholinesterase (AChE) is a serine protease, with hydrolytic activity, concentrated in the synaptic cleft at mainly neuromuscular junctions and cholinergic brain synapses, where its activity serves to terminate synaptic transmission.
AChE has a very high catalytic activity - each molecule of AChE degrades about 25000 molecules of acetylcholine (ACh) per second, approaching the limit allowed by diffusion of the substrate.
The active site of AChE comprises 2 subsites - the anionic site and the esteratic subsite.
The anionic subsite accommodates the positive quaternary amine of acetylcholine as well as other cationic substrates and inhibitors.
The esteratic subsite, where acetylcholine is hydrolyzed to acetate and choline, contains the catalytic triad of three amino acids: serine200, histidine 440 and glutamate 327. These three amino acids are similar to the triad in other serine proteases except that the glutamate is the third member rather than asparate.
(Source: Wikipedia. Keyword: Acetylcholinesterase)


Acetilcolina
A deficiency of acetylcholine caused by selective loss of cholinergic neurons in the cerebral cortex, nucleus basalis, and hippocampus is recognized as one of the early pathophysiologic features of Alzheimer's disease associated with memory loss and cognitive deficits. Because the resultant cortical deficiency of this neurotransmitter is believed to account for some of the clinical manifestations of mild to moderate dementia, enhancement of cholinergic function with an anticholinesterase agent, such as tacrine or donepezil, is one of the pharmacologic approaches to treatment. Because widespread degeneration of multiple central neuronal systems eventually occurs in patients with Alzheimer's disease, potentially beneficial effects of anticholinesterase agents theoretically would diminish as the disease process advances and fewer cholinergic neurons remain functioning.
[American Society of Health System Pharmacists.
AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 1293]
SIDE EFFECTS
The acetylcholinesterase inhibitors improve cognitive performance in manifest dementia; these substances, however, also influence the quality of sleep, and particularly the quality and amount of dreams. Therefore was observed a clear-cut relationship between the occurrence of nightmares and an evening dose of donepezil in patients with DAT; none of these patients reported nightmares when donepezil was taken in the morning. This event suggest that the activation of the visual association cortex during REM sleep is enhanced by donepezil, a mechanism most likely facilitating the development of nightmares in patients with DAT.
[Singer M et al; Nervenarzt 76 (9): 1127-9 (2005). Available from, as of July 6, 2009:]
TOXICITY
Donepezil hydrochloride is contraindicated in patients with known hypersensitivity to this drug or to piperidine derivatives.
Because of their pharmacological action, cholinesterase inhibitors may have vagotonic effects on the sinoatrial and atrioventricular nodes. This effect may manifest as bradycardia or heart block in patients both with and without known underlying cardiac conduction abnormalities. Syncopal episodes have been reported in association with the use of donepezil hydrochloride.
Through their primary action, cholinesterase inhibitors may be expected to increase gastric acid secretion due to increased cholinergic activity. Therefore, patients should be monitored closely for symptoms of active or occult gastrointestinal bleeding, especially those at increased risk for developing ulcers, eg, those with a history of ulcer disease or those receiving concurrent non-steroidal anti-inflammatory drugs (NSAIDS)
[US Natl Inst Health; DailyMed. Current Medication Information for Donepezil Hydrochloride (donepezil hydrochloride) tablets, film coated (May 2008). Available from, as of June 29, 2009:http://dailymed.nlm.nih.gov.offcampus.dam.unito.it/dailymed/drugInfo.cfm?id=7596