Francesco Olivero

Minoxidil is an antihypertensive vasodilator drug which also slows or stops hair loss and promotes hair regrowth: it is available for the treatment of androgenic alopecia.
DESCRIPTION
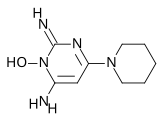
Minoxidil occurs as a white or off-white, odourless, crystalline solid which is readily soluble in propylene glycol or ethanol, soluble in water to the extent of 2 mg/mL and is almost insoluble in acetone, chloroform or ethyl acetate.
The chemical name for minoxidil is 6-piperidin-1-ylpyrimidine-2,4-diamine 3-oxide (MW = 209.25).
With Finasteride is currently the only drug approved by the FDA for the treatment of androgenetic alopecia.
PHARMACOLOGY and PHARMACODYNAMICS
Minoxidil has been shown to stimulate hair growth in males and females with androgenic alopecia (however, the exact mechanism of action is not known). Minoxidil needs to be applied once or the recommended twice daily. The regrowth can be observed after approximately 4 or more months of use and is variable among patients. Upon discontinuation of treatment with Minoxidil, new hair growth stops and restoration of pretreatment appearance may occur within 3-4 months.
Minoxidil administered orally for the treatment of hypertension has a direct peripheral vasodilator effect which reduces elevated systolic and diastolic blood pressure by decreasing peripheral vascular resistance (Minoxidil reduced supine diastolic blood pressure by 20 mmHg or to 90 mmHg or less in approximately 75% of patients ). Reduction of peripheral arteriolar resistance and the associated fall in blood pressure induces sympathetic, vagal inhibitory, and renal homeostatic mechanisms, including an increase in renin secretion, which lead to increased heart rate and cardiac output, and salt and water retention. Minoxidil does not interfere with vasomotor reflexes and therefore does not produce orthostatic hypotension.
MOLECULAR MECHANISM
Minoxidil is a potassium channel opener, causing hyperpolarization of cell membranes and it is also a vasodilator. It is speculated that, by widening blood vessels and opening potassium channels, it allows more oxygen, blood and nutrients to the follicle.
Human hair follicles contain two forms of ATP-sensitive potassium channels, only one of which is sensitive to minoxidil .
This can also cause follicles in the telogen phase to shed, usually soon to be replaced by new, thicker hairs in a new anagen phase.

The hair growth cycle is composed by three phases: Anagen= growth phase; Catagen= degradation phase; Telogen= resting phase. Periods of growth (anagen) between two and eight years are followed by a brief period, two to four weeks, in which the follicle is almost totally degraded (catagen). The resting phase (telogen) then begins and lasts two to four months. Shedding of the hair occurs only after the next growth cycle (anagen) begins and a new hair shaft begins to emerge. On average 50-100 telogen hairs are shed every day.
Minoxidil stimulates hair follicles and growth, but does not reduce Dihydrotestosterone (DHT) or the enzyme responsible for its accumulation around the hair follicle, 5-alpha reductase, which is the primary mediator of male pattern baldness in genetically susceptible individuals. Therefore, when treatment is stopped, the DHT has its expected effect of shrinking and ultimately destroying the genetically predisposed hair follicles.
PHARMACOKINETICS
Minoxidil is poorly absorbed from normal intact skin, with an average of approximately 1.7% of the total applied dose ultimately reaching the systemic circulation. In contrast, minoxidil is almost completely absorbed from the gastrointestinal tract following oral administration of minoxidil tablets. The metabolic biotransformation of minoxidil absorbed following topical application has not been fully determined. The active form of the drug appears to be a sulfated metabolite, minoxidil sulfate. Orally administered minoxidil is metabolised predominantly by conjugation with glucuronic acid at the N-oxide position in the pyrimidine ring but also by conversion to more polar products. Minoxidil does not bind to plasma proteins and its renal clearance corresponds to the glomerular filtration rate (125ml/min). Minoxidil and its metabolites are haemodialysable, and are excreted principally in the urine. It does not cross the blood brain barrier.
SIDE EFFECTS
General:
Minoxidil topical is generally well tolerated. Dermatological adverse events are the only side effects reported more commonly as compared to placebo.
Cardiovascular
Edema , salt and water retention, pericardial effusion, pericarditis, tamponade, tachycardia, and angina have been reported with oral minoxidil. Patients with underlying heart disease may be at increased risk for these or other cardiovascular adverse effects.
Rarely, cardiovascular side effects have included edema, chest pain, blood pressure changes, palpitations, and changes in pulse rate with minoxidil topical therapy.
Local
Despite low levels of systemic absorption, in one study of 35 men using either topical minoxidil 2% twice a day or placebo for 6 months, minoxidil was associated with cardiac changes, such as significant increases in left ventricular end-diastolic volume, cardiac output, and left ventricular mass.
Systemic side effects are uncommon since very little minoxidil is absorbed after topical application. In one study, the serum levels after 2.5 mg twice a day oral versus 2% twice a day topical minoxidil were 32.8 and 1.7 ng per mL, respectively.
Exacerbation of hair loss/alopecia has been reported.
Dermatologic
Rare cases of generalized hypertrichosis have been associated with topically applied minoxidil. Eczema, irritant dermatitis, and allergic contact dermatitis have also been reported.
There have been cases of allergic reactions to the nonactive ingredient propylene glycol, which is found in some topical solution especially if they are galenic.
h3. Hypersensivity
Nonspecific allergic reactions, hives, allergic rhinitis, facial swelling, and sensitivity to minoxidil topical have rarely been reported.
Nervous system
Headache, dizziness, faintness, and light-headedness have been reported with minoxidil topical.
Gastrointestinal
Diarrhea, nausea, and vomiting have been reported during treatment with minoxidil topical.
Ocular
Ocular
Visual disturbances, including decreased visual acuity, have been reported.
Urogenital
Rare cases of erectile dysfunction.
INTOXICATION
Accidental intoxications in children are frequent but most of them are without serious consequences. We describe herein the case of a young girl who drank 100 mg of a topical hair lotion with minoxidil. On arrival, she had no symptoms except flush on the face and ears. Four and half hours after ingestion, tachycardia appeared with a pulse above 170 beats per min with hypotension at 76/24 mmHg. The heart rate remained between 170 and 190 beats per min for 12 h and then lowered to between 140 and 160 beats per min. Thirty-six hours after ingestion, the heart beat was at 140 beats per min. Minoxidil is a strong vasodilator used first in the 1970s for severe hypertension. It produces hypotension by direct arteriolar vasodilatation. Only a few cases of minoxidil intoxication have been described in the literature, including only one pediatric case. This young boy had only tachycardia of 160 beats per min for 40 h. Most serious cases have been described in adults. They suffered long-lasting tachycardia, hypotension, and ECG changes. Most patients need a bolus of normal saline fluid and some with hemodynamic problems need vasoactive drugs such as dopamine and/or phenylephrine. All patients need to be under medical supervision for a long time because of the product's very long action.