DESCRIPTION
Atropa Belladonna, is a poisonous plant also called deadly nightshade. It grow up in the mountain area in North Europe, Africa and West Asia. This plant is a perennial herb classified in the Solanaceae family, has an inclusive height among the 70-150 cm, the flowers are green-purple, that bloom during the summer, the berries are black. Despite the pleasant aspect, the whole plant results to be poisonous and provokes, if ingested, thirst, vomit, hallucinations followed by convulsion and death.

Hystory
The Atropa Belladonna takes the name from its lethal effects and the use in the field of the cosmetics. Atropa, in the Greek mythology, it is tied up to the Goya Atropo(Goya) that cut the life's thread. The name Belladonna takes its origin in the Renaissance because Italian' dames used this plant to improve the colour of the skin and to give brigtness to the eyes. The substance atropine is extracted by this plant which was prepared the ointment called witches'whisper. This substance is also very well know in medicine since the time of Ippocrate in 400 B.C. The atropine has a particular charm that has involved in the centuries witches, poets, writers, medical scientists and alchemists. In Middle Ages, these plants are used in the rituals to meet Satana because they have powers both hypnotics that aphodisiacs.
Fascinated by this substance a teacher German, Will Enrich Peuckert, in 1960, found a witchcraft recipe drawn by the book “Magic Naturalis” of Giambattista and, following the instructions,Peuckert prepares an ointment that uses on himself. It falls in catalepsy and in a twenty hours deep sleep during which he was tormented by horrible visions: monsters, infernal landscapes, diabolic beings and satanic creatures.
MODE OF ACTION
Its roots, leaves and fruits contain alkaloids like atropine, scopolamine. Alkaloids usually derive from vegetable, they possess one or more nitrogen (N) atoms and they have strong physiological actions on human and animals, they are strongly basic and very poisonus(Herbal Extracts and Phytochemicals: Plant Secondary Metabolites, 2011). Atropine acts by interfering with the transmission of nerve impulses by acetylcholine,a small organic molecule liberated at the nerve ending as a neurotrasmitter, because it is an antagonist of muscarinic receptors located into the central nervous sistem, smooth muscles, cardiac muscle and exocrine glands. This is a competitive type of antagonism, and the effect can be overcome with high concentration of acetylcholine. Muscarinic receptors has a pocket with a aspartate recidue responsable of the ionic bond with atropine, this site of bond is the same used by acetylcholine (Farmacologia)
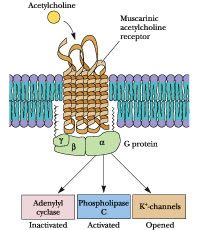
All muscarinic receptors are G-protein coupled receptors, there are five subtypes of muscarinic AChRs based on pharmacological activity. M1, M3 and M5 receptors cause the activation of phospholipase C, generating two secondary messengers (IP3 and DAG) eventually leading to an intracellular increase of calcium, while M2 and M4 inhibit adenylate cyclase, thereby decreasing the production of the second messenger cAMP and leading to an inhibition of voltage-gated Ca2+ channels. Atropine binds these receptors and inhibit their activaction by acetylcholine, the systemic effect of this action is the inhibition of the parasympathetic nervous system.
BIOSYNTHESIS
The biosynthesis of Atropine starting from L-Phenyalanine first undergoes a transamination forming Phenypyruvic Acid which is then reduced to Phenyl-lactic Acid. Coenzyme A then couples Phenyl-Lactic Acid with Tropine forming Littorine, which then undergoes a radical rearrangement initiated with a P450 enzime forming Hyoscyamine Aldehyde. A dehydrogenase then reduces the aldehyde to a primary alcohol making Hyoscyamine, which upon racemization forms atropine.

EFFECT
(Atropa Belladonna intoxication: a case report, 2012)
Atropa Belladonna is associated with an acute intoxication called anti-cholinergic toxidrome.
The ingestion of 10 berries would be toxic to an adults, an 3 berries for a child. The risk of intoxication is children in important for the possible confusion with other berries. Atropa Belladonna poisoning has central and peripheral nervous system effect, they are dose dependent. Central nervous effect are ataxia, disorientation, short-term memory loss, confusion, hallucinations, psychosis, agitated delirium, seizures, coma, respiratory failure or cardiovascular collapse. The peripheral effect are mydriasis with cycloplegia, dry mucous membranes, hyperreflexia, flushed skin, diminished bowel sounds or ileus, urinary retention, tachycardia, and hypertension or hypotension. This anti-cholinergic toxidrome can be confused with post-traumatic brain damage and acute psychosis. This effect are dose dependent, the therapeutic dose of atropine is 0.5-2mg and this administration causes decrease in heart rate by vagal stimulation, dryness of mouth, inhibition of swearing, mild dylation of pupils. Great doses of 2mg cause all typical effect of anti-cholinergic toxidrome like:
- 5mg: difficult swallowing, headache, dry hot skin, reduced intestinal peristalsis
- 10mg: pulse rapid and weak, mydriasis (iris obliterated), hot dry scarlet skin, ataxia, allucination, coma, delirium.

ABSORPTION, METABOLISM, EXCRETION
After oral administration, the atropine is quickly absorbed by the GI system except the stomach. Absorpition result more rapid after with intramuscular of subcutaneous injection than GI because less drug needed for the same effect. After the absorpiton, up to 50% of atropine is bound to protein. The liver metabolized atropine and the excretion is managed by the kidneys (80-90%) and feces. The Clearance is 5,9 ml/min/kg, and the substance half life is 4,3 h. ( Anticholinergic side-effects of drugs in elderly people,2000)
THERAPEUTICAL USE
Anticholinergic drugs like atropine are used in anesthesia because they reduce the activity of salivatory glands and correct tha vagal-induced bradycardia. The atropine is suitable for the care of a lot of GI problems like abnormal intestinal motility, bile-stone, irritate bowel syndrome. In oculistic, it is used for its ability to induce mydriasis and reduced ocular accomodation because the atropine stops the answers to the cholinergic stimulation of the muscle sphincter of the iris and the ciliary muscle. If the atropine is administered locally, the ocular effect is considerable and the accomodation returns normal after 7-12 days (Farmacologia). In 2006 anticholinergic drugs were used also for the initial treatment of early idiopathic Parkinson’s disease (PD) (Drug therapy in patients with Parkinson’s disease,2012)