Davide Preve
Lorena Salusso
INTRODUCTION
The use of plastic dishes to consume warm food can make to meaningfully increase the risk of kidney stones.
The new Taiwanese study ( a pilot study) included 16 healthy volunteers (age range, 20-27 years) to consume 500 mL of hot noodle soup (initial temperature, 90°C) served in melamine bowls in the morning of October 2011. They are collected from each participant 1 spot urine sample immediately before and at 2-hour intervals for 12 hours after consuming the noodle soup. This experiment simulated the natural situation; thus, not all participants provided urine samples at every 2-hour interval. However, all urine samples from all participants were collected after consumption for 12 hours. Postconsumption mean urinary melamine concentrations, corrected for urinary creatinine, initially increased sharply, peaked at 4 to 6 hours, and then declined sharply for 2 hours and then less steeply for the remainder of the monitoring period (eFigure 1). They therefore investigated if consumption of hot noodle soup served in melamine bowls would increase total urinary melamine excretion. Three weeks later, the participants consumed the same kind of soup but the type of bowl they used was reversed. Urine samples were collected again.
The study was published online Jan. 21 in the journal JAMA Internal Medicine.
"A Crossover Study of Noodle Soup Consumption in Melamine Bowls and Total Melamine Excretion in Urine. 2013"
archinte.jamanetwork
nlm.nih.gov
WHAT IS MELAMINE
Melamine is an organic compound that is often combined with formaldehyde to produce melamine resin, a synthetic polymer that is fire resistant and heat tolerant. The resin is a versatile material that has a highly stable structure. Its uses include whiteboards, floor tiles, kitchenware, fire retardant fabrics and commercial filters. Melamine can be easily molded while warm but will set into a fixed form, which makes it suitable for certain industrial applications. This compound is considered safe for its normal uses, but food products that are contaminated with it can be unsafe for consumption.
Resin
This type of resin is made by mixing melamine with formaldehyde, and sometimes urea, under heat and pressure. The substances begin to polymerize, and are forced into a mold to create the desired shape. Under pressure, melamine releases water, which could make the plastic unstable if it is not removed. The materials finish polymerizing and create a finished product.Melamine resin is known as a thermoset plastic because it is fixed after molding. If exposed to enough heat, it will decompose. For this reason, this type of dishware should not be exposed to high temperatures such as those in an oven or microwave. This type of resin also is difficult to recycle.
Foam
Foam products also can be made out of melamine. This foam has a distinctive structure composed of stacked bubble shapes that are extremely hard and therefore can easily clean a wide variety of substances. Melamine foam is marketed under a variety of commercial names, including several sponge-like products that are known for removing scuffs and dirt from a wide range of surfaces.
Heat Resistance
Melamine also plays a role in a wide range of flame-resistant materials. These include textiles that are used in upholstery and the uniforms worn by firemen. Thermal liners and heat-resistant gloves and aprons also are made using melamine.
Filters
Some filters also are made out of melamine. The material is porous and will admit substances to pass through but can be used to filter out particles of a particular size. These filters are capable of handling a high capacity, can be used in hot environments and are extremely efficient.
Others
In the present event, melamine contamination has been found in a number of different brands of powdered infant formula, in one brand of a frozen yogurt dessert and in one brand of canned coffee drink. All these products were most probably manufactured using ingredients made from melamine-contaminated milk.
www.wisegeek.org
Molecular Formula: C3-H6-N6

Physical Property
. pKa Dissociation Constant 5
. Melting Point 354 deg C
. Water Solubility 3240 mg/L
. Molecular Weight 126.12
. Color/Form Monoclinic prisms; monoclinic colorless prisms or crystals; white, monoclinic crystals
http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+108-78-1
CHEMICAL PROPERTIES
Melamine and related triazines are able to form self-assembling, high molecular weight complexes via organized intramolecular networks of hydrogen bonds and π-π aromatic ring stacking (Seto & Whitesides, 1990; Whitesides, Mathias & Seto, 1991).
It is commercially synthesized from urea with an intermediate step producing cyanic acid. The reaction also results in the formation of other byproducts, including cyanuric acid, ammeline, and ammelide.

Biomolecules possessing similar cyclic imide structures with the ability to form hydrogen bond networks are common. Uracil, riboflavin, barbituric acid and uric acid each possess imide groups known to interact with melamine in self-associating complexes. Triazine hydrogen bond networks may be subject to disruption by acid–base equilibria or high temperature.
CINETIC

Melamine is rapidly excreted in the urine (90% of the administered dose in 24 h)
KIDNEY STONES

Studies on animals in the past few years showed that the combination of melamine and cyanuric acid was the main cause of crystals in the tubules. Previous study showed that the composition of stones was primarily melamine and uric acid. A clinicopathological study by Lam et al.1 showed a strong correlation between renal stone size and urinary melamine concentration (Figure: Correlation of urine melamine-to-creatinine ratio to the largest diameter of the stone)
POSSIBLE MECHANISM OF MELAMINE NEPHROTOXICITY
Melamine is the principal culprit in this disease, but several studies in animals and humans suggest several susceptibility factors. Biopsy specimens in animals have reported several different pathologic sequelae. Melamine nephrotoxicity is characterized by nephrolithiasis, acute kidney injury (AKI), or both. The mechanism of melamine nephrotoxicity is still unknown, but clues are available from animal studies. Melamine can precipitate in distal renal tubules. Histopathologic specimens from affected cats and dogs show characteristic intratubular green radial crystals and crystalluria. The morphology and histochemical staining pattern is distinct from calcium oxalate or calcium phosphate crystals. Dilated distal tubule contains fragmented or globular dense green melamine-cyanuric acid crystals with tubular epithelial necrosis and regeneration.. Large aggregates of characteristic crystals distend a distal tubule. Tubular epithelial cells partially cover the crystals, with plasma cells in the fibrotic interstitium. Large crystals in the renal papilla were also described. Some affected animals suffered from acute kidney injury (AKI), whereas others developed chronic kidney disease (CKD). Melamine-associated AKI in animals was characterized by necrosis of distal tubular cells and mild inflammation. Chronic toxicity showed larger crystals, interstitial inflammation, and fibrosis. Similar lesions were found on autopsy with experimentally induced AKI after exposure to melamine and cyanuric acid. AKI in these animals is presumed to be due, at least inpart, to increased intrarenal pressure developing after intratubular obstruction from crystal deposition and distal tubular necrosis. Autopsy from cats and dogs with AKI also suggested that they may have had AKI due to corticomedullary hemorrhage or inflammation.
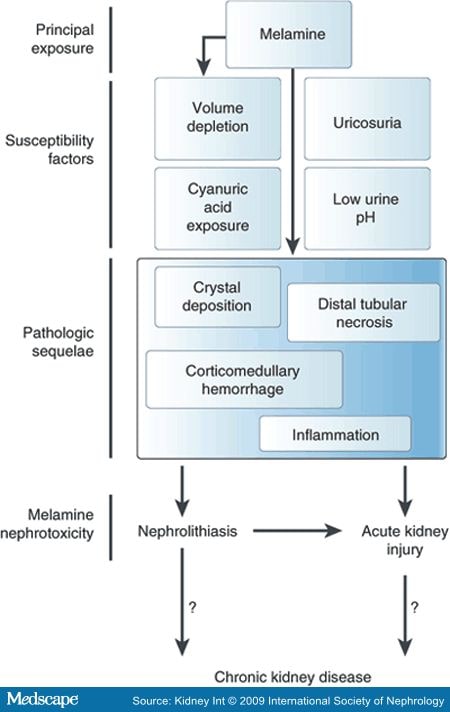
 Interestingly, when tested directly, neither melamine nor cyanuric acid alone causes nephrolithiasis or AKI in animals but both are necessary and sufficient to cause melamine-cyanurate co-crystallization both in vivo and in vitro.] Cyanuric acid, melamine's 'partner-in-crime,' is a white, odorless solid, which has been used for many years as a chlorine-stabilizing agent in swimming pools.] Cyanuric acid forms an insoluble lattice structure when mixed with melamine at pH 5.8.] This feature may also explain why intratubular crystal deposition is seen in the distal nephron in animals with this disease, unlike in oxalosis where crystal deposition is predominantly in the proximal nephron. Therefore, low urinary pH may be a risk factor for crystal deposition and AKI in humans. A compound exposure to melamine and cyanuric acid may determine which patients are susceptible to nephrolithiasis and possibly to AKI. Levels of cyanuric acid in foods have not been systematically and rigorously tested, but is approved that melamina is partially metabolized by intestinal (Jutzi et al. (1982)) bacterial flora in its derivatives (mono-, di, e trideaminati/idrossilati ammelina, ammelide and cyanur acid). it's possible that the cyanuric acid is maked bioavailable by bacteria process in the human intestin and it's excreted by urine (Cheng et al., 2005).
Interestingly, when tested directly, neither melamine nor cyanuric acid alone causes nephrolithiasis or AKI in animals but both are necessary and sufficient to cause melamine-cyanurate co-crystallization both in vivo and in vitro.] Cyanuric acid, melamine's 'partner-in-crime,' is a white, odorless solid, which has been used for many years as a chlorine-stabilizing agent in swimming pools.] Cyanuric acid forms an insoluble lattice structure when mixed with melamine at pH 5.8.] This feature may also explain why intratubular crystal deposition is seen in the distal nephron in animals with this disease, unlike in oxalosis where crystal deposition is predominantly in the proximal nephron. Therefore, low urinary pH may be a risk factor for crystal deposition and AKI in humans. A compound exposure to melamine and cyanuric acid may determine which patients are susceptible to nephrolithiasis and possibly to AKI. Levels of cyanuric acid in foods have not been systematically and rigorously tested, but is approved that melamina is partially metabolized by intestinal (Jutzi et al. (1982)) bacterial flora in its derivatives (mono-, di, e trideaminati/idrossilati ammelina, ammelide and cyanur acid). it's possible that the cyanuric acid is maked bioavailable by bacteria process in the human intestin and it's excreted by urine (Cheng et al., 2005).
!!
The effect of pH on the solubility of the melamine–cyanurate hydrogen-bonded complex was recently determined (Figure 4) (Tolleson,2008). The solubility of the complex increased markedly as the pH decreased to pH 3.5 and the concentration of melaminium cation increased. The solubility of melamine–cyanurate increased marginally as the pH increased to pH 7.5.
At neutral pH, the affinity of melamine for uric acid was found to be 29-fold weaker than that for cyanuric acid. Melamine–urate exhibited up to 6.3-fold tighter binding under acidic conditions in comparison with its affinity at pH 7.0 (Tolleson, 2008) . Chickens, which normally excrete larger amounts of uric acid than mammals, were exposed to melamine in preliminary USFDA studies. These studies showed that spherulites, presumably composed of melamine–urate crystals, dissolved rapidly in formalin fixative and its can not be identified (R. Reimschussel, personal communication, 2008).
Melamine and related amine analogs have also natriuretic properties ( in dogs at a dose of 125 mg/kg), and thus, may exacerbate AKI by leading to pre-renal azotemia in susceptible animals or individuals. (11 February 2009 “Melamine nephrotoxicity: an emerging epidemic in an era of globalization” Vivek Bhalla1, Paul C Grimm2, Glenn M Chertow1 and Alan C Pao)
www.nature.com
TOXICITY
Melamine is described as being "Harmful if swallowed, inhaled or absorbed through the skin. Chronic exposure may cause cancer or reproductive damage. Eye, skin and respiratory irritant." However, the short-term lethal dose is on a par with common table salt with an LD50 of more than 3 grams per kilogram of bodyweight. U.S. Food and Drug Administration (FDA) scientists explained that when melamine and cyanuric acid are absorbed into the bloodstream, they concentrate and interact in the urine-filled renal microtubules, then crystallize and form large numbers of round, yellow crystals, which in turn block and damage the renal cells that line the tubes, causing the kidneys to malfunction.
The European Union set a standard for acceptable human consumption (Tolerable Daily Intake) of melamine at 0.2 mg per kg of body mass (previously 0.5 milligrams), Canada declared a limit of 0.35 mg and the US FDA's limit was put at 0.063 mg daily (previously 0.63 mg). The World Health Organization's food safety director estimated that the amount of melamine a person could stand per day without incurring a bigger health risk, the "tolerable daily intake" (TDI), was 0.2 mg per kg of body mass.
Acute toxicity
Melamine is reported to have an oral LD50 of 3248 mg/kg based on rat data. It is also an irritant when inhaled or in contact with the skin or eyes. Melamine cyanurate, commonly used as a fire retardant, could be more toxic than either melamine or cyanuric acid alone. A toxicology study in animals conducted after recalls of contaminated pet food concluded that the combination of melamine and cyanuric acid in diet does lead to acute renal failure in cats. A 2008 study produced similar experimental results in rats and characterized the melamine and cyanuric acid in contaminated pet food from the 2007 outbreak. A 2010 study from attributed renal failure in humans to uric acid stone accumulation after ingestion of melamine resulting in a rapid aggradation of metabolites such as cyanuric acid diamide (ammeline) and cyanuric acid.
Chronic toxicity
Ingestion of melamine may lead to reproductive damage, or bladder or kidney stones, which can lead to bladder cancer.
http://en.wikipedia.org/wiki/Melamine
There is inadequate evidence in humans for the carcinogenicity of melamine. There is sufficient evidence in experimental animals for the carcinogenicity of melamine under conditions in which it produces bladder calculi. In making the overall evaluation, the Working Group noted that a non-DNA reactive mechanism by which melamine produced urinary bladder tumors in male rats occurred only under conditions in which calculi were produced. Melamine is not classifiable as to its carcinogenicity to humans (Group 3).
toxnet.nlm.nih.gov
RESULTS STUDY ' A CROSSOVER STUDY OF NOODLE SOUP CONSUMPTION IN MELAMINE BOWLS AND TOTAL MELAMINE EXCRETION IN URINE'
archinte.jamanetwork.com
Melamine tableware may release large amounts of melamine when used to serve high-tmperature foods.
Total melamine levels in urine for 12 hours after eating the soup was 8.35 micrograms when the participants ate out of the melamine bowls versus about 1.3 micrograms when they ate out of ceramic bowls. The difference was statistically significant. The significance was probably due to the high melamine excretion (carry-over effect).