DEFINITION
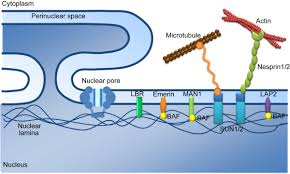
LEM domain-containing protein 3 (LEMD3), also known as MAN1, is an integral protein in the inner nuclear membrane (INM) of the nuclear envelope.
It is encoded by the LEMD3 gene and was first identified after it was isolated from the serum of a patient with a collagen vascular disease.
| ID | MAN1_HUMAN |
| DESCRIPTION | Inner nuclear membrane protein Man1 (LEM domain-containing protein 3) |
| SUBCELLULAR LOCATION | Nucleus;nuclear inner membrane;multi-pass membrane protein |
| TISSUE SPECIFICITY | Heart, brain, placenta, lung, liver and skeletal muscle |
| SIMILARITY | Contains 1 LEM domain |
(Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis,2004)
THE GENE
LEMD3 (or MAN1) is a 82.3-kDa protein with an amino-terminal domain followed by two hydrophobic segments and a carboxyl-terminal tail.
The MAN1 gene contains seven protein-coding exons and is assigned to human chromosome 12q14.

Its mRNA is approximately 5.5 kilobases and is detected in several different cell types.
There are domains within MAN1 common to other proteins.
There are over 40 human EST sequences identical to portions of MAN1 and numerous expressed sequence tags with similar sequences from other organisms. From nucleotide 829 to 1077, the nucleotide sequence of MAN1 is 100% identical to the sequence of a previously reported CpG island. CpG islands are short stretches of DNA containing a high density of nonmethylated CpG dinucleotides, predominantly associated with coding regions.
(MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin,2000)
CHEMICAL STRUCTURE
The human MAN antigens termed MAN1 is a 82.3-kDa protein with an amino-terminal domain followed by two hydrophobic segments and a carboxyl-terminal tail.
Hydropathy analysis shows that MAN1 contains two hydrophobic amino acid stretches . These sequences meet the criteria for transmembrane segments. There is no hydrophobic amino-terminal signal sequence, strongly suggesting that if the first hydrophobic stretch in MAN1 is a transmembrane segment, the domain of approximately 470 amino acids that precedes it would be synthesized facing the cytoplasm and face the nucleoplasm when the protein is in the inner nuclear membrane. Hydropathy analysis further predicts that MAN1 has a carboxyl-terminal domain of approximately 100 amino acids following the second hydrophobic segment.
Protein sequence analysis reveals that MAN1 shares a conserved globular domain of approximately 40 amino acids, which we term the LEM module, with inner nuclear membrane proteins Lamina-associated Polypeptide 2 and Emerin.
The LEM module is also present in two proteins of Caenorhabditis elegans.


(MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin,2000)
Protein and Aminoacid Percentages


CELLULAR FUNCTIONS
MAN1 is an integral protein of the inner nuclear membrane that shares the LEM module with other proteins of this subcellular localization.
Immunoelectron and immunofluorescence microscopy have demonstrated that the MAN antigens are exclusively localized to the nuclear envelope, most likely the inner nuclear membrane .
Experiments
FLAG-tagged MAN1 expressed from its cDNA in transfected cells was targeted to the nuclear envelope, probably the inner nuclear membrane. Detection of the expressed protein with anti-FLAG antibodies in cells examined by confocal immunofluorescence microscopy showed that it was localized exclusively to the nuclear envelope in most cells.
In a few cells in which MAN1 appeared to be expressed at very high levels, weak labeling of the endoplasmic reticulum was observed.
A mostly exclusive nuclear envelope localization in transfected cells, along with “back-up” into the endoplasmic reticulum at high expression levels, is characteristic for integral proteins of the inner nuclear membrane . In occasional overexpressing cells, intranuclear fluorescence was observed, which may have resulted from invaginations of the inner nuclear membrane or a nonmembrane degradation fragment of MAN1 that was transported to the nucleus.

Functions
The LEMD3 protein helps control two chemical signaling pathways called the transforming growth factor beta (TGF-ß) pathway and the Bone Morphogenic Protein (BMP) pathway.
Signaling through these pathways turns on proteins called Smads, which attach to specific areas of DNA to activate certain genes.
C-terminal domain of human MAN1 binds to Smad2 and Smad3 and antagonizes signaling by transforming growth factor-beta (TGF-beta).
In a yeast two-hybrid screen using the C-terminal domain of MAN1 as bait, eight positive clones were obtained that encoded Smad3. In direct two-hybrid assays, this portion of MAN1 bound to Smad2 and Smad3. In glutathione-S-transferase precipitation assays, the C-terminal domain of MAN1 bound to Smad2 and Smad3 under stringent conditions. Antibodies against MAN1 were able to co-immunoprecipiate Smad2 from cells, demonstrating that they reside in the same complex in vivo. TGF-beta treatment stimulated transcription from a reporter gene in control cells, but reporter gene stimulation was significantly inhibited in cells overexpressing MAN1 or its C-terminal domain but not its N-terminal domain. TGF-beta-induced cell proliferation arrest was also inhibited in stable cell lines overexpressing MAN1. These results show that the nuclear envelope regulates a signal transduction pathway and have implications for how mutations in nuclear envelope proteins cause different human diseases.
The TGF-ß and BMP pathways regulate various cellular processes:
- Cell growth and proliferation;
(MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling,2005)
DISEASES
LEMD3 mutation is found in Osteopoikilosis, Buschke-Ollendorff syndrome (BOS) and Melorheostosis, which are disorders characterized by increased bone density. The occurrence of one or more of these phenotypes in the same individual or family suggests that these entities might be allelic.
(Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis,2004)
LEMD3 mutation was also found in Dermatofibrosis Lenticularis Disseminata.
(Familial cutaneous collagenomas resulting from a novel mutation in LEMD3,2007)
Authors
Enrico Spinoni
Stefano Tappero