Francesca Borda
Giulia Pavan
DESCRIPTION
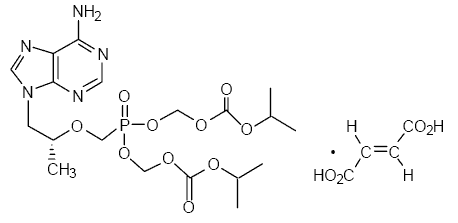
Tenofovir disoproxil fumarate (a prodrug of tenofovir), marketed by Gilead Sciences under the trade name Viread®, belongs to a class of antiretroviral drugs known as nucleotide analogue reverse transcriptase inhibitors (nRTIs), which block reverse transcriptase, an enzyme crucial to viral production in HIV-infected people. In vivo tenofovir disoproxil fumarate is converted to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5’-monophosphate.
Molecular Weight: 635.5166.
Empirical formula: C19H30N5O10P • C4H4O4
CATEGORIES
Anti-HIV Agents
Reverse Transcriptase Inhibitors
INDICATION
Tenofovir is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older.
Tenofovir is currently in late-stage clinical trials for the treatment of hepatitis B in adults and pediatric patients 12 years of age and older.
FARMACODYNAMIC
Tenofovir disoproxil fumarate is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. Tenofovir requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate.
Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.

FARMACOKINETICS PROPERTIES
ABSORTION:VIREAD is a water soluble diester prodrug of the active ingredient tenofovir. The oral bioavailability of tenofovir from VIREAD in fasted subjects is approximately 25%. Following oral administration of a single dose of VIREAD 300 mg to HIV-1 infected subjects in the fasted state, maximum serum concentrations (Cmax) are achieved in 1.0 ± 0.4 hrs. Cmax and AUC values are 0.30 ± 0.09 µg/mL and 2.29 ± 0.69 µg∙hr/mL, respectively.
In a single-dose bioequivalence study conducted under non-fasted conditions (dose administered with 4 oz. applesauce) in healthy adult volunteers, the mean Cmax of tenofovir was 26% lower for the oral powder relative to the tablet formulation. Mean AUC of tenofovir was similar between the oral powder and tablet formulations.
DISTRIBUTION:In vitro binding of tenofovir to human plasma or serum proteins is less than 0.7 and 7.2%, respectively, over the tenofovir concentration range 0.01 to 25 µg/mL.
The volume of distribution at steady-state is 1.3 ± 0.6 L/kg and 1.2 ± 0.4 L/kg, following intravenous administration of tenofovir 1.0 mg/kg and 3.0 mg/kg.
METABOLISM AND ELIMINATION:In vitro studies indicate that neither tenofovir disoproxil nor tenofovir are substrates of CYP enzymes.
Following IV administration of tenofovir, approximately 70–80% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Following single dose, oral administration of VIREAD, the terminal elimination half-life of tenofovir is approximately 17 hours. After multiple oral doses of VIREAD 300 mg once daily (under fed conditions), 32 ± 10% of the administered dose is recovered in urine over 24 hours.
Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
EFFECTS OF FOOD ON ORAL ABSORTION
Administration of VIREAD 300 mg tablets following a high-fat meal increases the oral bioavailability, with an increase in tenofovir of approximately 40% and an increase in Cmax of approximately 14%. However, administration of VIREAD with a light meal did not have a significant effect on the pharmacokinetics of tenofovir when compared to fasted administration of the drug.
MOLECULAR MECHANISM
Tenofovir inhibits the activity of HIV reverse transcriptase by competing with the natural substrate deoxyadenosine 5’-triphosphate and, after incorporation into DNA, by DNA chain termination.
Specifically, the drugs are analogues of the naturally occurring deoxynucleotides needed to synthesize the viral DNA and they compete with the natural deoxynucleotides for incorporation into the growing viral DNA chain. However, unlike the natural deoxynucleotides substrates, NRTIs and NTRTIs (nucleoside/tide reverse transcriptase inhibitors) lack a 3'-hydroxyl group on the deoxyribose moiety. As a result, following incorporation of an NRTI or an NtRTI, the next incoming deoxynucleotide cannot form the next 5'-3' phosphodiester bond needed to extend the DNA chain. Thus, when an NRTI or NtRTI is incorporated, viral DNA synthesis is halted, a process known as chain termination. All NRTIs and NtRTIs are classified as competitive substrate inhibitors.
POSOLOGY
For the treatment of HIV-1 or chronic hepatitis B: the dose is one 300 mg VIREAD tablet once daily taken orally, without regard to food.
In the treatment of chronic hepatitis B, the optimal duration of treatment is unknown.
Safety and efficacy in pediatric patients with chronic hepatitis B weighing less than 35 kg have not been established.
For the treatment of HIV-1 in pediatric patients 2 years of age and older, the recommended oral dose of VIREAD is 8 mg of tenofovir disoproxil fumarate per kilogram of body weight (up to a maximum of 300 mg) once daily administered as oral powder or tablets.
Significantly increased drug exposures occurred when VIREAD was administered to subjects with moderate to severe renal impairment. Therefore, the dosing interval of VIREAD tablets 300 mg should be adjusted in patients with baseline creatinine clearance below 50 mL/min.
SIDE EFFECTS
The most common side effects associated with tenofovir include:
nausea, vomiting, diarrhea, and asthenia.
Less frequent side effects include:
hepatotoxicity, abdominal pain, and flatulence.
Tenofovir has also been implicated in causing renal toxicity, particularly at elevated concentrations.
Tenofovir can cause acute renal failure, Fanconi syndrome, proteinuria or tubular necrosis. These side effects are due to accumulation of the drug in proximal tubules.
ARTICLE
THE RULE of TENOFOVIR IN HIV PROPHYLAXIS
“Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals”
Charles I Okwundu , Olalekan A Uthman, Christy AN Okoromah
Editorial Group: Cochrane HIV/AIDS Group
Published Online: 11 JUL 2012
BACKGROUND:
More than 30 years into the global HIV/AIDS epidemic, infection rates remain alarmingly high, with over 2.7 million people becoming infected every year. There is a need for HIV prevention strategies that are more effective. Oral antiretroviral pre-exposure prophylaxis (PrEP) in high-risk individuals may be a reliable tool in preventing the transmission of HIV.
OBJECTIVES:
To evaluate the effects of oral antiretroviral chemoprophylaxis in preventing HIV infection in HIV-uninfected high-risk individuals.
SECTION CRITERIA:
Randomised controlled trials that evaluated the effects of any antiretroviral agent or combination of antiretroviral agents in preventing HIV infection in high-risk individuals
RESULTS:
We identified 12 randomised controlled trials that meet the criteria for the review. Six were ongoing trials, four had been completed and two had been terminated early. Six studies with a total of 9849 participants provided data for this review. The trials evaluated the following: daily oral tenofovir disoproxil fumarate (TDF) plus emtricitabine (FTC) versus placebo; TDF versus placebo and daily TDF-FTC versus intermittent TDF-FTC. One of the trials had three study arms: TDF, TDF-FTC and placebo arm. The studies were carried out amongst different risk groups, including HIV-uninfected men who have sex with men, serodiscordant couples and other high risk men and women.
Overall results from the four trials that compared TDF-FTC versus placebo showed a reduction in the risk of acquiring HIV infection (RR 0.49; 95% CI 0.28 to 0.85; 8918 participants). Similarly, the overall results of the studies that compared TDF only versus placebo showed a significant reduction in the risk of acquiring HIV infection (RR 0.33; 95% CI 0.20 to 0.55, 4027 participants). There were no significant differences in the risk of adverse events across all the studies that reported on adverse events. Also, adherence and sexual behaviours were similar in both the intervention and control groups.
AUTHOR’S CONCLUSIONS:
Finding from this review suggests that pre-exposure prophylaxis with TDF alone or TDF-FTC reduces the risk of acquiring HIV in high-risk individuals including people in serodiscordant relationships, men who have sex with men and other high risk men and women.
THE RENAL FUNCTION AFTER TENOFOVIR THERAPY
“The rate of recovery in renal function when patients with HIV infection discontinue treatment with tenofovir”
J Young1,*, Q Wang1, CA Fux2, E Bernasconi3, H Furrer4, P Vernazza5, A Calmy6, M Cavassini7, R Weber8,
M Battegay9, HC Bucher1,9 and theSwiss HIV Cohort Study†
Article first published online: 18 MAR 2014
Published by Wiley online library
OBJECTIVES:
Tenofovir is associated with reduced renal function. It is not clear whether patients can be expected to fully recover their renal function if tenofovir is discontinued.
METHODS:
We calculated the estimated glomerular filtration rate (eGFR) for patients in the Swiss HIV Cohort Study remaining on tenofovir for at least 1 year after starting a first antiretroviral therapy regimen with tenofovir and either efavirenz or the ritonavir-boosted protease inhibitor lopinavir, atazanavir or darunavir. We estimated the difference in eGFR slope between those who discontinued tenofovir after 1 year and those who remained on tenofovir.
RESULTS:
A total of 1049 patients on tenofovir for at least 1 year were then followed for a median of 26 months, during which time 259 patients (25%) discontinued tenofovir. After 1 year on tenofovir, the difference in eGFR between those starting with efavirenz and those starting with lopinavir, atazanavir and darunavir was – 0.7 [95% confidence interval (CI) −2.3 to 0.8], −1.4 (95% CI −3.2 to 0.3) and 0.0 (95% CI −1.7 to 1.7) mL/min/1.73 m2, respectively. The estimated linear rate of decline in eGFR on tenofovir was −1.1 (95% CI −1.5 to −0.8) mL/min/1.73 m2per year and its recovery after discontinuing tenofovir was 2.1 (95% CI 1.3 to 2.9) mL/min/1.73 m2 per year. Patients starting tenofovir with either lopinavir or atazanavir appeared to have the same rates of decline and recovery as those starting tenofovir with efaviren
CONCLUSIONS:
If patients discontinue tenofovir, clinicians can expect renal function to recover more rapidly than it declined.
REFERENCES
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000419/WC500105683.pdf
- http://en.wikipedia.org/wiki/Tenofovir
-ww.rxlist.com/viread-drug.htm
- https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_002455_035565_RCP.pdf&retry=0&sys=m0b1l3