Palmitoleic acid is an omega-7 monounsaturated fatty acid (16:1n-7) that is a common constituent of the glycerides of human adipose tissue. It is present in all tissues but, in general, found in higher concentrations in the liver. It is biosynthesized from palmitic acid by the action of the enzyme Stearoyl-CoA desaturase-1.



Stearoyl-CoA desaturases have a high Glu/Gln ratio pointing to activity in environments with high oxygen concentration, like nerves or peri-portal liver localization.
It has also signaling functions as a member of adipocytes signaling molecules lipokines.
openknowledgemaps
 palmitoleic+acid+and+desaturase
palmitoleic+acid+and+desaturase
 palmitoleic+acid+and+weight+loss
palmitoleic+acid+and+weight+loss
The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease
Palmitoleic acid reduces intramuscular lipid and restores insulin sensitivity in obese sheep
 Palmitoleic acid and adipose - Results from Quertle
Palmitoleic acid and adipose - Results from Quertle
Adipose tissue palmitoleic acid is inversely associated with nonfatal acute myocardial infarction in Costa Rican adults.
 Palmitoleic acid - Results from Quertle
Palmitoleic acid - Results from Quertle
Hormonal Modulation of Pheromone Detection Enhances Male Courtship Success. 2017
SUMMARY: During the lifespans of most animals, reproductive maturity and mating activity are highly coordinated. In Drosophila melanogaster, for instance, male fertility increases with age and older males are known to have a copulation advantage over young ones. The molecular and neural basis of this age-related disparity in mating behavior is unknown. Here we show that the Or47b odorant receptor is required for the copulation advantage of older males. Notably, the sensitivity of Or47b neurons to a stimulatory pheromone, palmitoleic acid, is low in young males but high in older ones, which accounts for older males’ higher courtship intensity. Mechanistically, this age-related sensitization of Or47b neurons requires a reproductive hormone, juvenile hormone, as well as its binding protein Methoprene-tolerant in Or47b neurons. Together, our study identifies a direct neural substrate for juvenile hormone that permits coordination of courtship activity with reproductive maturity to maximize male reproductive fitness.
Insulin protects pancreatic acinar cells from palmitoleic acid-induced cellular injury. 2014
Acute pancreatitis is a serious and sometimes fatal inflammatory disease where the pancreas digests itself. The non-oxidative ethanol metabolites palmitoleic acid (POA) and POA-ethylester (POAEE) are reported to induce pancreatitis caused by impaired mitochondrial metabolism, cytosolic Ca2+ ([Ca2+]i) overload and necrosis of pancreatic acinar cells. Metabolism and [Ca2+]i are linked critically by the ATP-driven plasma membrane Ca2+-ATPase (PMCA) important for maintaining low resting [Ca2+]i. The aim of the current study was to test the protective effects of insulin on cellular injury induced by the pancreatitis-inducing agents, ethanol, POA, and POAEE. Rat pancreatic acinar cells were isolated by collagenase digestion and [Ca2+]i was measured by fura-2 imaging. An in situ [Ca2+]i clearance assay was used to assess PMCA activity. Magnesium green (MgGreen) and a luciferase-based ATP kit were used to assess cellular ATP depletion. Ethanol (100 mm) and POAEE (100 μm) induced a small but irreversible Ca2+ overload response but had no significant effect on PMCA activity. POA (50–100 μm) induced a robust Ca2+ overload, ATP depletion, inhibited PMCA activity, and consequently induced necrosis. Insulin pretreatment (100 nm for 30 min) prevented the POA-induced Ca2+ overload, ATP depletion, inhibition of the PMCA, and necrosis. Moreover, the insulin-mediated protection of the POA-induced Ca2+ overload was partially prevented by the phosphoinositide-3-kinase (PI3K) inhibitor, LY294002. These data provide the first evidence that insulin directly protects pancreatic acinar cell injury induced by bona fide pancreatitis-inducing agents, such as POA. This may have important therapeutic implications for the treatment of pancreatitis.
 palmitoleic+acid+and+muscle
palmitoleic+acid+and+muscle
Palmitoleic acid induces the cardiac mitochondrial membrane permeability transition despite the presence of L-carnitine, 2015. Fulltext
The aim of this study was to elucidate the activity of L-carnitine in the prevention of the palmitoleic acid-induced mitochondrial membrane permeability transition and cytochrome c release using isolated cardiac mitochondria from rats. Palmitoleoyl-CoA-induced mitochondrial respiration was not accelerated by L-carnitine treatment, and this respiration was slightly inhibited by oligomycin, which is an inhibitor of ATP synthase. Despite pretreatment with L-carnitine, the mitochondrial membrane potential decreased and mitochondrial swelling was induced by palmitoleoyl-CoA.
It is known that sudden cardiac death is associated with excess free fatty acids [1], [2], although fatty acids are the major exogenous energy substrate in the healthy heart [3]. Indeed, arrhythmias cardiac apoptosis, and mitochondrial damage of the heart have all been reported to be associated with excess free fatty acids [4], [5], [6]. Fatty acids enhance energy production through β-oxidation in the mitochondria, and l-carnitine, which is synthesized from the amino acids lysine and methionine or obtained from dietary sources, is essential in this pathway, because the inner membrane of the mitochondria does not transport fatty acids without the action of l-carnitine [7]. The finding in experimental animals and human studies that the failing myocardium has a low content of l-carnitine supports the concept that cardiovascular disease is often accompanied by relative l-carnitine insufficiency. Of note, l-carnitine treatment was observed to decrease arrhythmias [8], [9], [10], and l-carnitine deficiency has been associated with heart failure [11]. l-Carnitine is associated with a 65% reduction in ventricular arrhythmias [12]. Although this is a high percentage, the remaining 35% risk suggests that the function of l-carnitine in removing toxic fatty acid intermediates might be imperfect.
Individual fatty acids may have varying effects on the development of arrhythmias. In recent studies, polyunsaturated fatty acids (n-3 and n-6) were shown to have anti-arrhythmic effects, whereas saturated fatty acids had pro-arrhythmic effects [2]. In contrast, monounsaturated fatty acids were considered to be positively associated with a risk of sudden cardiac death in an age-adjusted model [2]. In addition, it has been reported that *palmitoleic acid (C16:1), a monounsaturated fatty acid, might induce cardiac arrhythmias *, [14], [15]. Palmitoleic acids are also known to be associated with multiple metabolic risk factors [16]. Although the toxic effects of palmitoleic acids in the heart have been shown in numerous studies [5], [13], [14], [17], [18], the effects of l-carnitine treatment on the palmitoleic acid-induced mitochondrial dysfunction in the heart are unclear. We hypothesized that l-carnitine might not fully prevent cardiac mitochondrial dysfunction due to increasing palmitoleic acid β-oxidation. The aim of this study was to determine the differential effects of palmitic and palmitoleic acids in the presence of l-carnitine on cardiac mitochondrial function using isolated mitochondria from rat hearts.
 palmitoleic+acid+and+oxidative+metabolism
palmitoleic+acid+and+oxidative+metabolism
Adipose tissue depot differences in adipokines and effects on skeletal and cardiac muscle. 2020
Furthermore, several bioactive lipids termed lipokines [palmitoleate (C16:1n7) or 12,13-diHOME] and microRNAs capsuled in exosomes (miR-27a, miR122, miR-130b, miR-155, miR-200a or miR-320d) secreted from white and brown adipocytes also influence the skeletal and cardiac muscle function.
 palmitoleic+acid+and+cardiolipin
palmitoleic+acid+and+cardiolipin
some negative effects of PAO (on tumor prostate cells)
some positive effects on cardiolipin
 palmitoleic+acid+and+pancreas
palmitoleic+acid+and+pancreas
Palmitoleate inhibits insulin transcription by activating the ERK1/2 pathway in rat pancreatic β-cells. 2017
In conclusion, the present study suggested that palmitoleate may induce insulin secretion and inhibit insulin mRNA expression in pancreatic β-cells.
 palmitoleic+acid+and+adipokines
palmitoleic+acid+and+adipokines
 palmitoleic+acid+and+adiponectin
palmitoleic+acid+and+adiponectin
 palmitoleic+acid+and+HMG-CoA+reductase
palmitoleic+acid+and+HMG-CoA+reductase
 palmitoleic+acid+and+ubiquinone
palmitoleic+acid+and+ubiquinone
 palmitoleic+acid+and+nerves
palmitoleic+acid+and+nerves
The Novel Perspectives of Adipokines on Brain Health. 2019
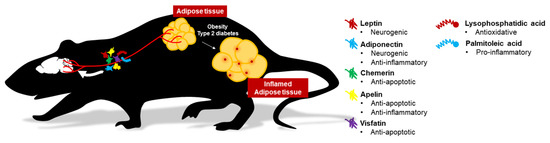
In this article, we have reviewed five adipokines (leptin, adiponectin, chemerin, apelin, visfatin) and two lipokines (palmitoleic acid and lysophosphatidic acid) on their roles involving in eating behavior, neurotrophic and neuroprotective factors in the brain.
Abnormal levels of palmitic acid have been documented in neurodegenerative
diseases [290–292] that are related to dysregulated palmitic acid biosynthesis.
Palmitoleic acid, a monounsaturated fatty acid, primarily originates from stearoyl-CoA desaturase 1-mediated
de novo lipogenesis from palmitic acid in humans [287]. Palmitoleic acid is highly abundant in serum
and adipose tissues [293]. cis-palmitoleate has been associated with increased insulin sensitivity
and decreased lipid accumulation in the liver [258]. Various animal models have illustrated that
cis-palmitoleate reduces the expressions of pro-inflammatory cytokines and adipokines in association
with metabolic syndromes [258,294,295]. In vitro study also shows that palmitoleic acid increases
lipolysis and lipase content in white adipose tissue in a PPARα-dependent manner [296]. Other
than adipose tissues, palmitoleic acid can enhance whole-body glucose disposal [297] and improve
circulating lipid profiles in both rodents and humans [298]. Therefore, palmitoleic acid is considered
a lipokine.
Palmitoylation is a sorting determinant for transport to the myelin membrane. 2005. Fulltext