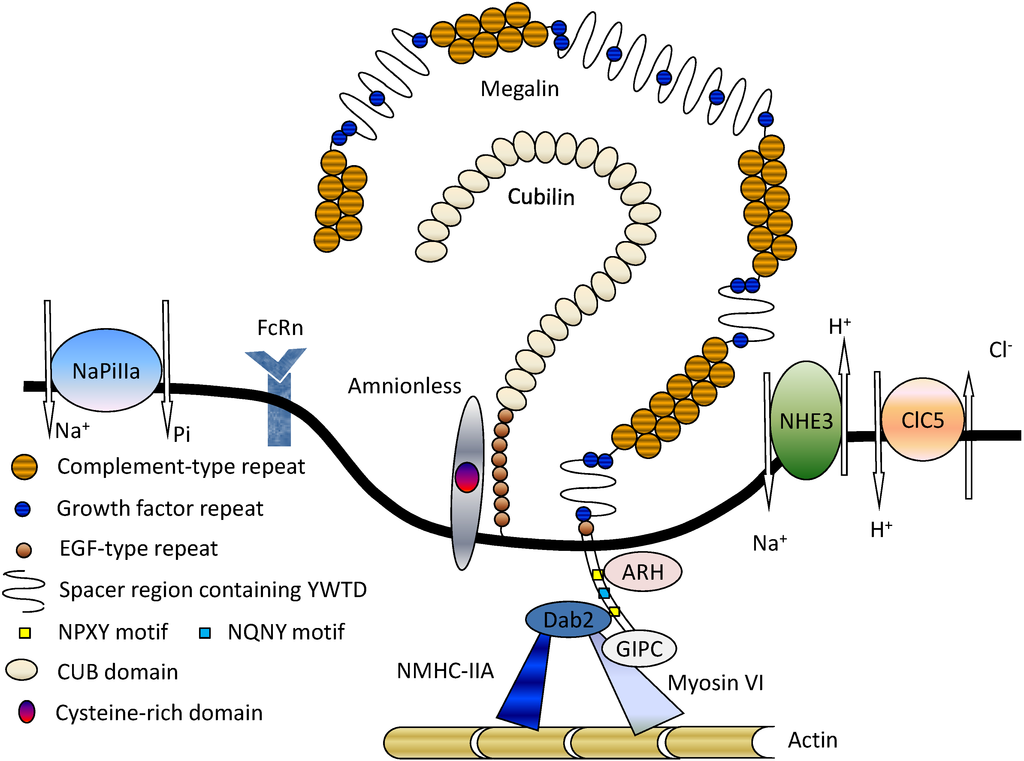
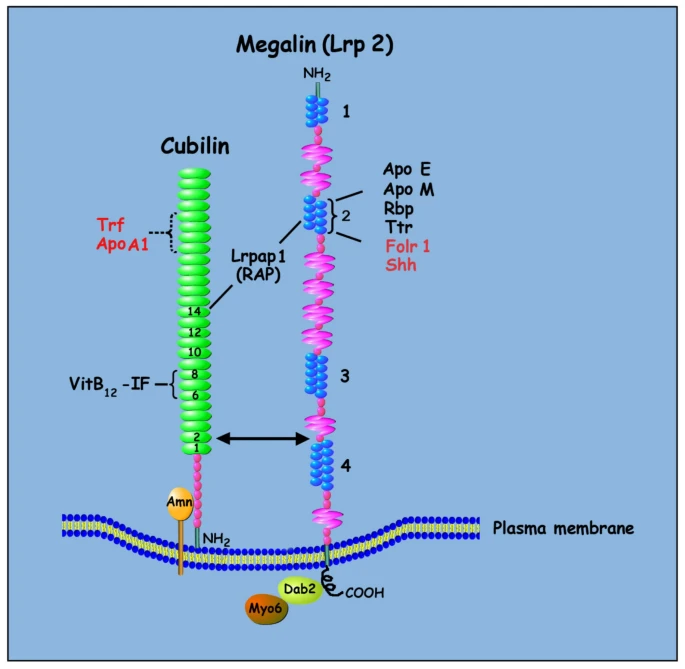
A cartoon diagram of the structure of the cubilin-amnionless-megalin multiligand receptor endocytic complex is shown. Megalin is a cell-surface receptor/transporter consisting of a large extracellular region, a single transmembrane domain, and a C-terminal cytoplasmic tail. The extracellular domain of megalin contains four clusters of lipoprotein receptor ligand-binding repeats (blue), growth factor repeats, an EGF repeat, and YWTD spacer regions. The second cluster of ligand-binding repeats has been identified as a common binding site for several ligands including apolipoprotein E (Apo E), apolipoprotein M (Apo M), retinol binding protein (Rbp), and transthyretin (Ttr). Megalin also binds the soluble form of the folate receptor (Folr1), and the morphogen sonic hedgehog (Shh). The cytoplasmic tail of megalin binds Dab2, a cytosolic adapter protein important for megalin-mediated endocytosis, and Dab2 binds and recruits Myo6 to clathrin-coated vesicles. The receptor-associated protein (Lrpap1; RAP) binds both megalin and cubilin. Cubilin is a peripheral membrane receptor comprised of a short amino terminal, eight EGF type domains, and 27 CUB domains (green). The amino-terminal end of cubilin is attached to the extracellular part of amnionless (Amn), and amnionless provides the transmembrane domain necessary for the anchoring and endocytic trafficking of cubilin. Cubilin ligands include transferring (Trf), albumin, hemoglobin, apolipoprotein A1 (ApoA1), and intrinsic factor (IF)-vitamin B12.
Structure
additional pictures
The Endocytic Receptor Megalin and its Associated Proteins in Proximal Tubule Epithelial Cells, 2014
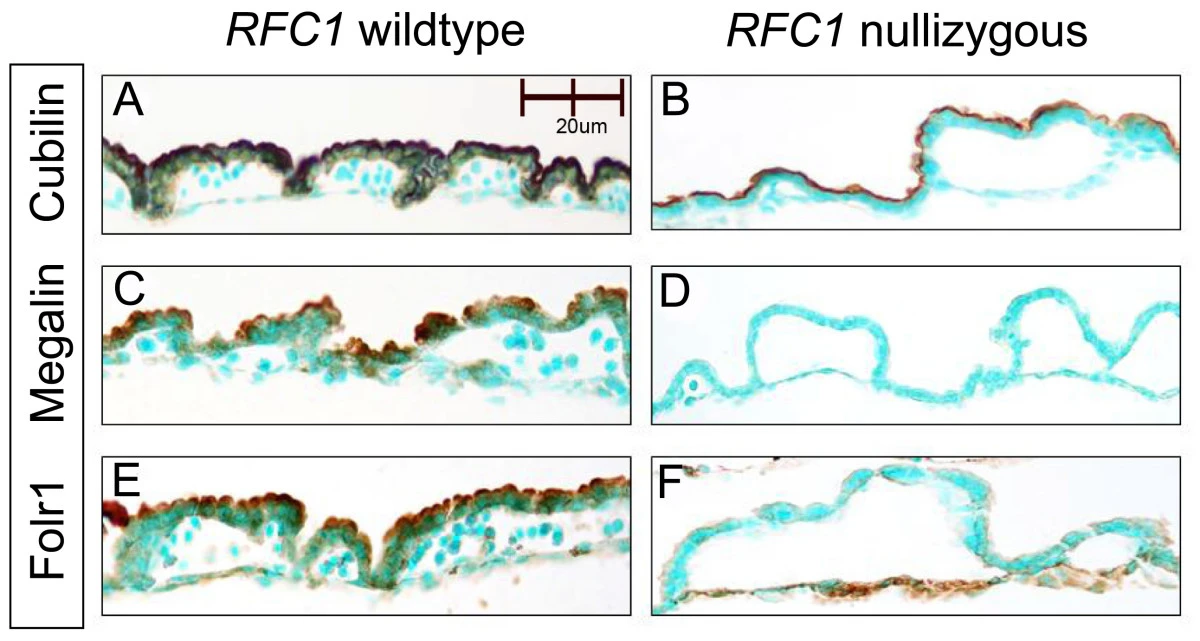
Microarray analysis of E9.5 reduced folate carrier (RFC1; Slc19a1) knockout embryos reveals altered expression of genes in the cubilin-megalin multiligand endocytic receptor complex, 2008
Additional figure
The amino-terminal region contains a potential palmitoylation site and an amphipathic alpha-helical structure with some similarity to the lipid-binding regions of apolipoproteins. Both are potential contributors to the anchoring of the receptor in the membrane
Ligands

Tissue distribution

Google Images Cubilin and Megalin
Vit B12 Absorption
Regulation
by TH4F
Microarray analysis of E9.5 reduced folate carrier (RFC1; Slc19a1) knockout embryos reveals altered expression of genes in the cubilin-megalin multiligand endocytic receptor complex, 2008