Tumor necrosis factor (TNF, cachexin or cachectin and formally known as tumor necrosis factor-alpha) is a cytokine involved in systemic inflammation and is a member of a group of cytokines that stimulate the acute phase reaction.

Cytokines are signaling molecules: which is their information content?
IL6 and TNF have low Tryptophan - Trp
IL6 has high Leu, an inducer of mTOR that activates protein synthesis
lipids pathways of inflammation
Protein Aminoacids Percentage (Width 700 px)


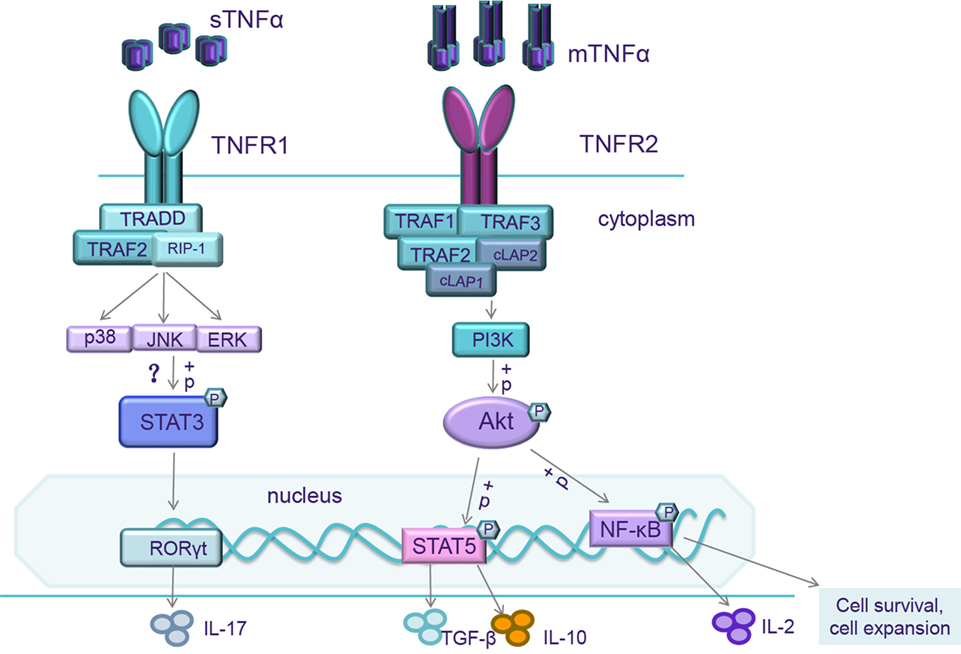
Role of TNF–TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications, 2018
- Tumor necrosis factor α (TNFα) is a pleiotropic cytokine which signals through TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2). Emerging evidence has demonstrated that TNFR1 is ubiquitously expressed on almost all cells, while TNFR2 exhibits a limited expression, predominantly on regulatory T cells (Tregs). In addition, the signaling pathway by sTNF via TNFR1 mainly triggers pro-inflammatory pathways, and mTNF binding to TNFR2 usually initiates immune modulation and tissue regeneration. TNFα plays a critical role in upregulation or downregulation of Treg activity. Deficiency in TNFR2 signaling is significant in various autoimmune diseases. An ideal therapeutic strategy for autoimmune diseases would be to selectively block the sTNF/TNFR1 signal through the administration of sTNF inhibitors, or using TNFR1 antagonists while keeping the TNFR2 signaling pathway intact. Another promising strategy would be to rely on TNFR2 agonists which could drive the expansion of Tregs and promote tissue regeneration. Design of these therapeutic strategies targeting the TNFR1 or TNFR2 signaling pathways holds promise for the treatment of diverse inflammatory and degenerative diseases.
TRAPS associata a glomerulonefrite
E' stato riportato il primo caso noto di glomerulonefrite rapidamente progressiva in un paziente con sindrome TRAPS (Tumor necrosis factor receptor-associated periodic syndrome). Quest'ultima, causata da mutazioni nei geni dei recettori per il TNF, la pi comune forma di febbre ricorrente di natura autosomica dominante; i sintomi della malattia comprendono artralgia, mialgia, sierositi, rash cutanei e dolori addominali. Dato che i problemi della regolazione del TNF-alfa sono stati implicati nella patogenesi sia della vasculite sistemica che dell'infiammazione glomerulare, la glomerulonefrite proliferativa potrebbe essere una caratteristica della sindrome TRAPS, ed andrebbe presa in considerazione nell'ampia gamma delle sue manifestazioni. (Arthritis Rheum 2008; 58: 3275
Transmembrane TNF alpha

- Transmembrane TNF-α (tmTNF-α) acts both as a ligand, delivering ‘forward signaling’ via TNFR, and as a receptor, transducing ‘reverse signaling’. The contradiction of available data regarding the effect of tmTNF-α on insulin resistance may be due to imbalance in both signals. Here, we demonstrated that high glucose-induced impairment of insulin-stimulated glucose uptake by 3T3-L1 adipocytes was concomitant with decreased tmTNF-α expression and increased soluble TNF-α (sTNF-α) secretion. However, when TACE was inhibited, preventing the conversion of tmTNF-α to sTNF-α, this insulin resistance was partially reversed, indicating a salutary role of tmTNF-α. Treatment of 3T3-L1 adipocytes with exogenous tmTNF-α promoted insulin-induced phosphorylation of IRS-1 and Akt, facilitated GLUT4 expression and membrane translocation, and increased glucose uptake while addition of sTNF-α resulted in the opposite effect. Furthermore, tmTNF-α downregulated the production of IL-6 and MCP-1 via NF-κB inactivation, as silencing of A20, an inhibitor for NF-κB, by siRNA, abolished this effect of tmTNF-α. However, tmTNF-α upregulated adiponectin expression through the PPAR-γ pathway, as inhibition of PPAR-γ by GW9662 abrogated both tmTNF-α-induced adiponectin transcription and glucose uptake. Our data suggest that tmTNF-α functions as an insulin sensitizer via forward signaling.
Transmembrane tumor necrosis factor-alpha sensitizes adipocytes to insulin, 2015
Antagonists of TNF
Keratinocyte growth factor protects murine hepatocytes from tumor necrosis factor-induced apoptosis in vivo and in vitro 2003;
Insulin and epidermal growth factor receptors contain the cysteine repeat motif found in the tumor necrosis factor receptor. 1995
J Periodontal Res. 2009 Feb;44(1):73-80.
Tumor necrosis factor-alpha-stimulated membrane type 1-matrix metalloproteinase production is modulated by epidermal growth factor receptor signaling in human gingival fibroblasts.
Smith PC, Guerrero J, Tobar N, Cáceres M, González MJ, MartÃnez J.
Faculty of Odontology, University of Chile, Santiago, Chile. patricio.smith@gmail.com
BACKGROUND AND OBJECTIVES: Membrane type 1-matrix metalloproteinase (MT1-MMP) is a collagenolytic enzyme involved in connective tissue remodeling. In periodontal tissues, either cytokines or growth factors regulate the production of proteolytic enzymes. Mice deficient in epidermal growth factor receptor (EGFR) show a reduced expression of MT1-MMP, suggesting that this receptor may play an important role in MT1-MMP production. The present study evaluated the role of the inflammatory cytokine tumor necrosis factor-alpha (TNF-alpha) and EGFR in the production of MT1-MMP in gingival fibroblasts. MATERIAL AND METHODS: Primary cultures of human gingival fibroblasts were cultured over plastic or a type I collagen matrix and stimulated with TNF-alpha and EGF. A selective EGFR inhibitor (AG1478) was used to interfere with this signaling pathway. Production of MT1-MMP and activation of proMMP-2 were studied using Western blot and gelatin zymography, respectively. Activation of EGFR signaling was assessed through immunoprecipitation and Western blot. Expression of EGFR ligands was determined through reverse transcriptase-polymerase chain reaction. RESULTS: Treatment of gingival fibroblasts cultured over a collagen matrix with TNF-alpha stimulated proMMP-2 activation and MT1-MMP production. However, after using AG1478, both responses were inhibited. Tumor necrosis factor-alpha induced EGFR transactivation and stimulated the expression of the mRNA for the EGFR ligands heparin binding-epidermal growth factor (HB-EGF) and transforming growth factor-alpha (TGF-alpha). CONCLUSIONS: The present study shows that TNF-alpha may stimulate MT1-MMP production through transactivation of EGFR. Tumor necrosis factor-alpha may also modulate the expression of the EGFR ligands TGF-alpha and HB-EGF. Production of MT1-MMP by TNF-alpha requires interaction with EGFR, suggesting that tissue remodeling is controlled by cross-communication between diverse signaling pathways in
Am J Physiol Gastrointest Liver Physiol. 2008 Aug;295(2):G285-93. Epub 2008 May 8.
Tumor necrosis factor inhibits ligand-stimulated EGF receptor activation through a TNF receptor 1-dependent mechanism.
McElroy SJ, Frey MR, Yan F, Edelblum KL, Goettel JA, John S, Polk DB.
Dept. of Pediatrics, Div. of Pediatric Gastroenterology, Hepatology, and Nutrition, 2215 Garland Ave., 1035 MRB IV, Nashville TN, 37232-0696, USA.
Tumor necrosis factor (TNF) and epidermal growth factor (EGF) are key regulators in the intricate balance maintaining intestinal homeostasis. Previous work from our laboratory shows that TNF attenuates ligand-driven EGF receptor (EGFR) phosphorylation in intestinal epithelial cells. To identify the mechanisms underlying this effect, we examined EGFR phosphorylation in cells lacking individual TNF receptors. TNF attenuated EGF-stimulated EGFR phosphorylation in wild-type and TNFR2, but not TNFR1, mouse colon epithelial (MCE) cells. Reexpression of wild-type TNFR1 in TNFR1 MCE cells rescued TNF-induced EGFR inhibition, but expression of TNFR1 deletion mutant constructs lacking the death domain (DD) of TNFR1 did not, implicating this domain in EGFR downregulation. Blockade of p38 MAPK, but not MEK, activation of ERK rescued EGF-stimulated phosphorylation in the presence of TNF, consistent with the ability of TNFR1 to stimulate p38 phosphorylation. TNF promoted p38-dependent EGFR internalization in MCE cells, suggesting that desensitization is achieved by reducing receptor accessible to ligand. Taken together, these data indicate that TNF activates TNFR1 by DD- and p38-dependent mechanisms to promote EGFR internalization, with potential impact on EGF-induced proliferation and migration key processes that promote healing in inflammatory intestin
Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. 2006
Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor alpha. 2004
Cancer Immunol Immunother. 1998 Nov;47(3):167-75.
Activation of EGF receptor family members suppresses the cytotoxic effects of tumor necrosis factor-alpha.
Hoffmann M, Schmidt M, Wels W.
Institute for Experimental Cancer Research, Tumor Biology Center, Freiburg, Germany.
Tumor necrosis factor (TNF)-alpha has a broad range of biological activities, which depend heavily on cell type and physiological condition. In a panel of human tumor cell lines we analyzed expression of the receptor tyrosine kinases EGFR, ErbB2 and ErbB3, and the response to TNF-alpha. Among the cell lines tested those resistant to TNF-alpha were found to express high levels of either EGFR, or ErbB2 and ErbB3. In TNF-sensitive breast and cervical carcinoma cells activation of EGFR or ErbB2 by the exogenous growth factors EGF and heregulin beta1 resulted in a significant increase in the number of cells surviving TNF-alpha treatment. In contrast, inhibition of EGFR activation in TNF-resistant breast carcinoma cells by the novel antagonistic anti-EGFR antibody 14E1 sensitized the cells to the cytotoxic effects of TNF-alpha. A bacterially expressed fusion protein consisting of a 14E1 single-chain (sc) Fv antibody fragment linked to human TNF-alpha retained TNF-alpha activity. This scFv(14E1)-TNF-alpha molecule localized specifically to EGFR on the surface of tumor cells and activated the NF-kappaB pathway in co-cultured T cells, as demonstrated by electrophoretic mobility shift assays.
J Interferon Cytokine Res. 1996 Apr;16(4):307-14.
Activation of epidermal growth factor receptor tyrosine phosphorylation by tumor necrosis factor correlates with loss of cytotoxic activity. 1996
Perez M, Donato NJ.
Department of Bioimmunotherapy, Box 41, 1515 Holcombe Boulevard Houston, TX 77030, USA.
TNF induces cytotoxicity in human tumor cells through a receptor-mediated process with unknown signaling characteristics. Evidence suggests that overexpression of transmembrane growth factor receptors with intrinsic tyrosine kinase activity may suppress the antiproliferative or cytotoxic activity of TNF, suggesting antagonism between these two signaling pathways in tumor cells. To investigate TNF cytotoxic signal transduction, ME-180 cervical carcinoma cell variants were isolated that expressed complete cytotoxic sensitivity (ME-180S) or resistance (ME-180R) to TNF but identical levels of p55 TNF receptor expression. ME-180R cells expressed threefold higher EGFR than the ME-180S cell line and TNF treatment stimulated EGFR tyrosine phosphorylation only in resistant cells. Activation of tyrosine phosphorylation in ME-180R cells was TNF concentration dependent and maximally stimulated (three- to-five-fold) after 10-15 minutes of treatment. Other tyrosine phosphoproteins were not affected by TNF incubation demonstrating specific TNF-stimulated tyrosine phosphomodulation of EGFR. Pretreatment with the tyrosine kinase inhibitor tryphostin before incubation with TNF resulted in partial reversal of TNF cytotoxic resistance in ME-180R cells and enhanced TNF responsiveness in ME-180S cells, suggesting a "protective" role for tyrosine phosphorylation in TNF-induced cytotoxicity. Together these results demonstrate that TNF-mediated tyrosine phosphorylation is differentially regulated in sensitive and resistant tumor cells and may play a critical role in the cytotoxic signaling process through differential expression or regulation of tyrosine protein kinases and phosphatases.
Sphingosine-1-phosphate-mediated-signaling-pathways
TRAF2 Has very low E3 ubiquitin ligase activity in the absence of sphingosine-1-phosphate. E3 ubiquitin ligase activity is strongly activated by cytoplasmic sphingosine-1-phosphate

TNF alpha and adiponectin
Do ACE-inhibitors suppress tumour necrosis factor-alpha production in advanced chronic renal failure? 1999
RESULTS Patients treated with ACE-inhibitors (n = 44) had significantly lower plasma TNF-alpha levels (18.5 +/- 1.2 vs. 26.6 +/- 2.2 pg mL-1; P < 0.01) and were less frequently malnourished, relative to 52 patients not treated with ACE-inhibitors.
ccccccccccccccccccccccccccccccccccccccccccccccccccc
cccccccccccccccccccccccccccccccccccccccccccccccccccccccc
cccccccccccccccccccccccccccccccccccccccccccccccccccccccccc