Tryptophan is an essential amino acid in the human diet. It is encoded in genetic code as the codon UGG.

Metabolism
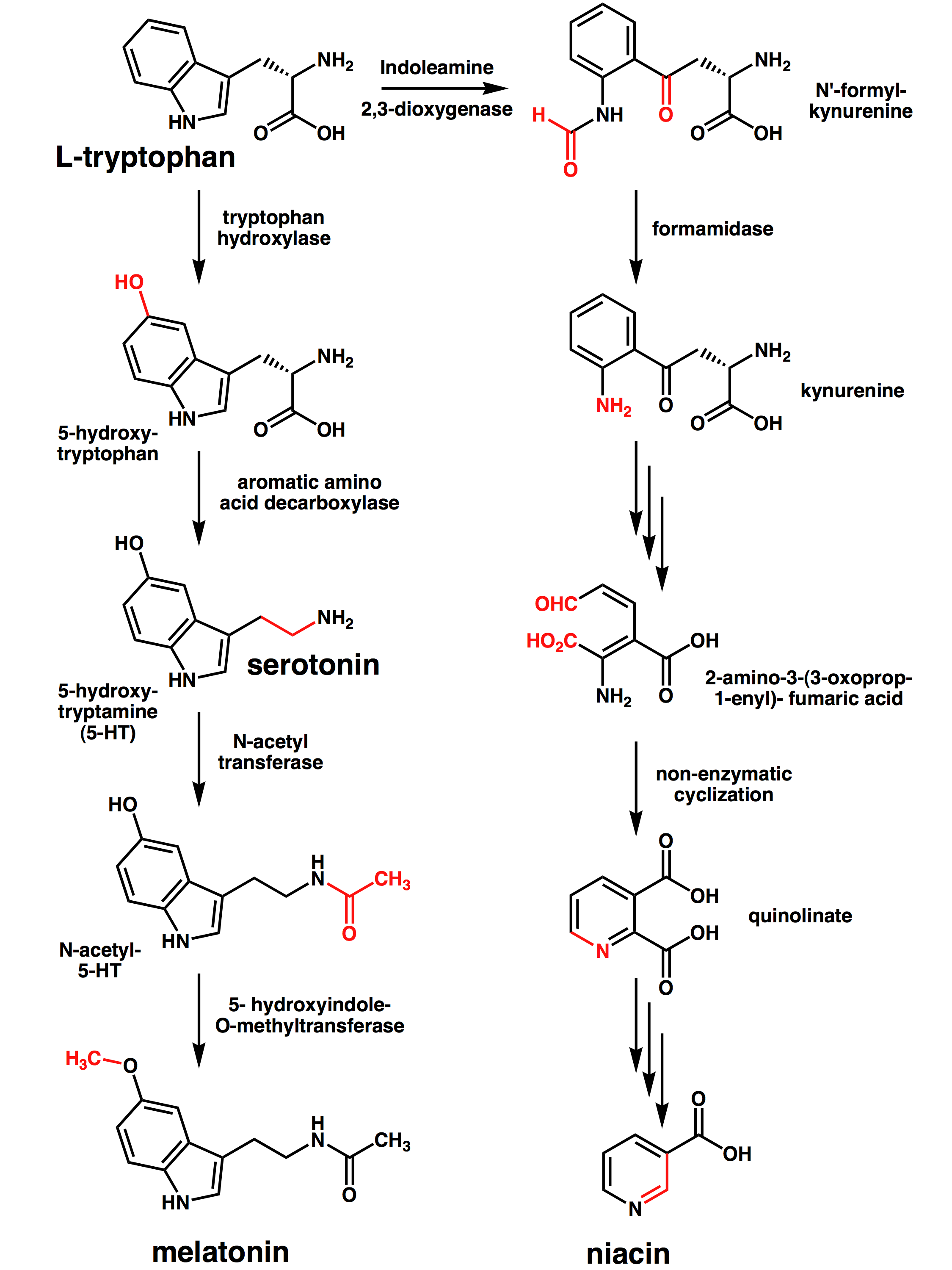
Of the dietary tryptophan that is not used in protein synthesis, 99% is metabolized along the kynurenine pathway (red arrows). Alternative pathways are the conversion of tryptophan to 5-hydroxytryptamine (5-HT) and then to melatonin, or to tryptamine and then to the kynuramines (or kynurenamines). 3-HAO, 3-hydroxyanthranilic acid oxidase; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; MAO, monoamine oxidase; QPRT, quinolinic-acid phosphoribosyl transferase; TDO, tryptophan 2,3-dioxygenase. (Endogenous kynurenines as targets for drug discovery and development, 2002) Figure
more details
Different kynurenine pathway enzymes limit quinolinic acid formation by various human cell types 1994
Indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) are heme oxygenase 2008
Final Products
Pathways A (1%)
Pathways B (99%)


- Picolinic acid
- Quinolic acid (Niacin -->NAD) (see details)


Endogenous kynurenines as targets for drug discovery and development 2002

Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? 2009
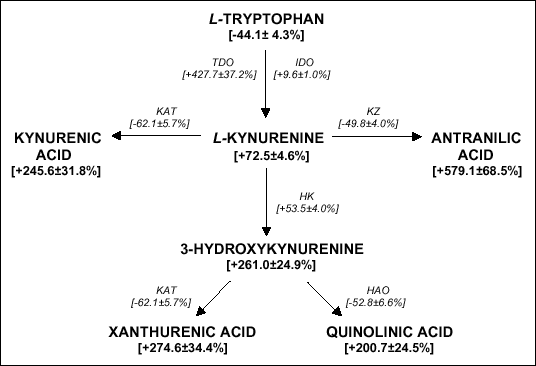
PERIPHERAL DISTRIBUTION OF KYNURENINE METABOLITES AND ACTIVITY OF KYNURENINE PATHWAY ENZYMES IN RENAL FAILURE
Cartoon IDO

Competition for Tryptophan
HIV
 HIV Tryptophan
HIV Tryptophan
*Which role for serum kynurenine in immune depression ? *
Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients2006
Tryptophan metabolism and vitamin B6 nutritional status in patients with schistosomiasis mansoni and in infected mice. 1992
Effects of oral contraceptives on tryptophan metabolism and vitamin B6 requirements in women. 1975
Interleukin-6/10 ratio as a prognostic marker of recurrence in patients with intermediate risk urothelial bladder carcinoma. 2007
Evaluation of cytokeratin-19 & cytokeratin-20 and interleukin-6 in Egyptian bladder cancer patients. 2002
Cytokines and major depression. 2005
Chlamidia
Dynamic diversity of the tryptophan pathway in chlamydiae: reductive evolution and a novel operon for tryptophan recapture 2002
Chlamidia and kinurenine
Tryptophan recycling is responsible for the interferon-γ resistance of Chlamydia psittaci GPIC in indoleamine dioxygenase-expressing host cells 2004
Red instead of black
Genes Cells. 2009 Feb;14(2):129-40. Epub 2008 Jan 6.
Abnormal red body coloration of the silkworm, Bombyx mori, is caused by a mutation in a novel kynureninase.
Meng Y, Katsuma S, Mita K, Shimada T.
Department of Agricultural and Environmental Biology, The University of Tokyo, Bunkyo-ku, Japan.
Larvae of the body color mutant red blood (rb) of the silkworm, Bombyx mori, display reddish skin whose hemolymph becomes red in air, whereas hemolymphs of normal strains become black during melanization. The irregular coloring was assumed to result from an abnormal accumulation of 3-hydroxykynurenine. However, the gene responsible for the rb phenotype is not yet known. Here, we provide evidence that the rb gene corresponds to a novel bacterial-type kynureninase gene, BmKynu. Kynureninase (KYNU) hydrolyzes kynurenine and 3-hydroxykynurenine to anthranilic acid and 3-hydroxyanthranilic acid, respectively. KYNU has been identified in microorganisms and animals but not in insects. Therefore, BmKynu is the first KYNU gene observed in insects. Our results clearly showed that a point mutation (T102I) in BmKYNU of the rb strain led to a marked decrease in KYNU activity, presumably resulting in abnormal accumulation of 3-hydroxykynurenine. Additionally, linkage analysis indicated that no recombination between rb and BmKynu was detected. We conclude that T102I in BmKYNU causes the red body coloration in the rb strain. Our study proves that B. mori has a unique side branch in the kynurenine pathway, distinctly different from other insects.