ChemPep Info on Aminoacids
Aminoacids spelling
Isoleucine
Leucine
Lysine
Methionine
Phenylalanine
Threonine
Tryptophan
Valine
Conditionally essential
Arginine
Cysteine
Glutamine
Glycine
Histidine
Proline
Tyrosine
Non essential amino acids
Alanine
Asparagine
Aspartate
Glutamate
Serine
Aminoacids Metabolism
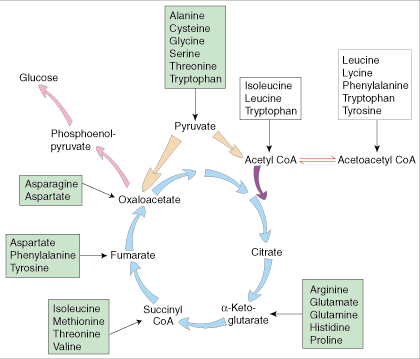
White boxes = chetogenic aminoacids
Green boxes = glucogenic aminoacids
Prenesti: Lisina e Arginina
Aminoacids DNA Codons

Aminoacids DNA Codons
Aminoacids DNA Code Structure
Aminoacids availability affects gene expression
Amino acid regulation of gene expression 2000
Effect on gene expression

Effect on mRNA translation

Effect on Autophagy
Intracellular protein catabolism and its control during nutrient deprivation and supply. 1987
Mortimore GE, Pösö AR.
Annu Rev Nutr. 1987;7:539-64.
The continuous turnover of intracellular protein and other macromolecules is a basic cellular process that serves, among other functions, to regulate cytoplasmic content and provide amino acids for ongoing oxidative and biosynthetic reactions during nutrient deprivation. The intensity of breakdown and pattern of regulation, though, vary widely among cells. Rat hepatocytes, for example, exhibit high absolute rates of proteolysis and regulatory effects that diminish during starvation, while corresponding responses in skeletal and cardiac muscle move in the opposite direction. It is also becoming apparent that effects of insulin and other acute regulatory agents on muscle breakdown are limited to nonmyofibrillar components. The latter may be sequestered and degraded within autophagic vacuoles, whereas myofibrillar proteins require an initial attack by calcium-dependent proteases in the cytosol. By contrast, most if not all of the breakdown of resident (long-lived) proteins as well as RNA in the hepatocyte can be explained by lysosomal mechanisms. The uptake of cytoplasmic components by lysosomes can be divided into two major categories, macroautophagy and micro- or basal autophagy. The first is induced by amino acid or insulin/serum deprivation. In the hepatocyte, amino acids alone can regulate this process almost instantaneously over two thirds of the full range of proteolysis, 4.5% to 1.5% per hour. Glucagon, cyclic AMP, and beta-agonists also stimulate macroautophagy in hepatocytes but have opposite effects in skeletal and cardiac myocytes. Basal autophagy differs from the macro type in that the cytoplasmic "bite" is smaller and sequestration is not acutely regulated. It is, however, adaptively decreased during starvation in parallel with absolute rates of basal turnover. Since endoplasmic reticulum comprises an appreciable fraction of the vacuolar content, volume sequestration would be compatible with the known heterogeneity of individual protein turnover if some proteins (or altered proteins) selectively bind to membranes. The amino acid control of macroautophagy in the hepatocyte is accomplished by a small group of direct inhibitors (Leu, Tyr/Phe, Gln, Pro, Met, Trp, and His) and the permissive effect of alanine whereas only leucine is involved in myocytes and adipocytes. Of unusual interest is the fact that the inhibitory amino acid group alone evokes responses in perfused livers that are identical to those of a complete plasma mixture at 0.5 and 4 times normal plasma levels but loses effectiveness almost completely at normal concentrations.(ABSTRACT TRUNCATED AT 400 WORDS)
Amino acids as regulators of proteolysis. 2003 J Nutr. 2003 Jun;133(6 Suppl 1):2052S-2056S. Kadowaki M, Kanazawa T.
- Proteolysis, as well as protein synthesis, is a major process that contributes to the body protein turnover. Despite the huge variety of proteases in the body, there are very few proteolytic systems contributing to the complete hydrolysis of proteins to amino acids. The autophagic-lysosomal pathway is responsible for bulk proteolysis, whereas the ubiquitin-proteasome pathway plays a significant role in the fine control of the degradation of specific proteins. Both systems can produce free amino acids as a final product, but only the autophagy system is physiologically controlled by plasma amino acids. Recently, the study of amino acids as regulators of macromolecular turnover has been focused on for their signal transduction mechanism. In autophagic proteolysis, several amino acids have a direct regulatory potential: Leu, Gln, Tyr, Phe, Pro, Met, Trp and His in the liver, and Leu in the skeletal muscle. These amino acids are recognized at the plasma membrane, indicating the possible existence of an amino acid receptor/sensor for their recognition and subsequent intracellular signaling. Another line of evidence has emerged that protein kinase cascades such as mTOR, Erk, eIF2alpha etc. may be involved in the regulation of autophagy, and that amino acids, in combination with insulin, may exert their effects through these pathways. From the viewpoint of amino acid safety, the contribution of proteolysis to possible adverse effects caused by excessive amino acid intake is not clear. At present, there is one report that excess glutamine at 10-fold the plasma level has an abnormal inhibitory effect on hepatic proteolysis, due to a lysosomotropic toxicity of ammonia derived from glutamine degradation. Whether this may lead to an adverse effect in humans remains to be clarified. Fulltext
Autophagy and orexin
orexin has 15 ala and 17 leu
 Papers cachexia orexin
Papers cachexia orexin
Nutrition. 2008 Sep;24(9):815-9.
Cachexia and neuropeptide Y.
Morley JE, Farr SA.
Division of Geriatric Medicine, Saint Louis University School of Medicine, St. Louis, Missouri, USA. morley@slu.edu
Abstract
Cachexia or wasting disease occurs commonly in diseases that have an overproduction of proinflammatory cytokines associated with them. The hallmarks of cachexia are loss of lean and adipose tissue, anorexia, anemia, memory disturbance, and sickness behavior. This review suggests that increased inducible nitric oxide synthase production in the hypothalamus leads to severe anorexia and that this is the pathway through which proinflammatory cytokines produce anorexia. Orexigenic peptides, such as neuropeptide, ghrelin, and orexin A, and anorectic peptides, such as leptin, produce their effects through neuronal nitric oxide synthase. Activation of neuronal nitric oxide synthase results in increased adenosine monophosphate kinase and a decrease in malonyl coenzyme A, leading to increased food intake.
Inflammation
Modifications of protein and amino acid metabolism during inflammation and immune system activation, Livestock Production Science 87 (2004) 37–45
Serum Aminoacids

Aminoacidscatabolism
"The metabolism of "surplus" amino acids. 2013":http://www.ncbi.nlm.nih.gov/pubmed/23107522
- For an adult in N balance, apart from small amounts of amino acids required for the synthesis of neurotransmitters, hormones, etc, an amount of amino acids almost equal to that absorbed from the diet can be considered to be "surplus" in that it will be catabolized. The higher diet-induced thermogenesis from protein than from carbohydrate or fat has generally been assumed to be due to increased protein synthesis, which is ATP expensive. To this must be added the ATP cost of protein catabolism through the ubiquitin-proteasome pathway. Amino acid catabolism will add to thermogenesis. Deamination results in net ATP formation except when serine and threonine deaminases are used, but there is the energy cost of synthesizing glutamine in extra-hepatic tissues. The synthesis of urea has a net cost of only 1·5 × ATP when the ATP yield from fumarate metabolism is offset against the ATP cost of the urea cycle, but this offset is thermogenic. In fasting and on a low carbohydrate diet as much of the amino acid carbon as possible will be used for gluconeogenesis - an ATP-expensive, and hence thermogenic, process. Complete oxidation of most amino acid carbon skeletons also involves a number of thermogenic steps in which ATP (or GTP) or reduced coenzymes are utilized. There are no such thermogenic steps in the metabolism of pyruvate, acetyl CoA or acetoacetate, but for amino acids that are metabolized by way of the citric acid cycle intermediates there is thermogenesis ranging from 1 up to 7 × ATP equivalent pe
NHEVNAT: an H+ V-ATPase electrically coupled to a Na+:nutrient amino acid transporter (NAT) forms an Na+/H+ exchanger (NHE), 2009
vvv
vvvv
vv