Giulia Benedetto Davide Michele Capone

DEFINITION
Tryptophan (IUPAC-IUBMB abbreviation: Trp or W; IUPAC abbreviation: L-Trp or D-Trp; sold for medical use as Tryptan) is one of the 20 standard amino acids , as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG. Only the L-stereoisomer of tryptophan is used in structural or enzyme proteins, but the D-stereoisomer is occasionally found in naturally produced peptides (for example, the marine venom peptide contryphan). The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as demonstrated by its growth effects on rats. Frederick Hopkins first reported the isolation of tryptophan in 1901 through hydrolysis of casein. From 600 grams of crude casein 4-8 grams of tryptophan can be obtained.

THE TRYPTOPHAN BIOSYNTHETIC PATHWAY
Tryptophan biosynthesis is a biologically expensive, complicated process. In fact, the products of four other pathways are essential contributors of carbon or nitrogen during tryptophan formation. Thus, the principal pathway precursor, chorismate, is also the precursor of the other aromatic amino acids, phenylalanine and tyrosine, as well as serving as the precursor of p- aminobenzoic acid and several other metabolites. In addition, glutamine, phosphoribosylpyrophosphate, and L-serine contribute nitrogen and/or carbon during tryptophan formation.

The seven reactions, involved in tryptophan formation are shown in the figure. Two of the tryptophan pathway enzymes often function as polypeptide complexes: anthranilate synthase, consisting of the TrpG and TrpE polypeptides, and tryptophan synthase, consisting of the TrpB and TrpA polypeptides.
Tryptophan synthase is an enzyme that catalyzes the final two steps in the biosynthesis of tryptophan. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyrodoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25-angstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channelling. The active sites of tryptophan synthase are allosterically coupled.

Tryptophan synthase is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from animalia. So humans have to take tryptophan from food. Tryptophan is a routine constituent of most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yoghurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, pumpkin, corn, spirulina and peanuts.
(Foods highest in Tryptophan)
TRYPTOPHAN METABOLISM
Tryptophan also functions as a biochemical precursor for the following compounds:
• Niacin is synthesized from tryptophan via kynirenine and quinolic acids as key biosynthetic intermediates
• Serotonin or 5-hydroxytryptamine (5-HT) (a neurotransmitter ), synthesized by a short metabolic pathway consisting of two enzymes: tryptophan hydroxylase (TPH) and amino acid decarboxylase (DDC). The TPH-mediated reaction is the rate-limiting step in the pathway. TPH has been shown to exist in two forms: TPH1, found in several tissues, and TPH2, which is a brain-specific isoform
• Serotonin, in turn, can be converted to melatonin (a neurohormone), via N-acetyltransferase and 5-hydrossyindole O methyltransferase activities

NIACIN
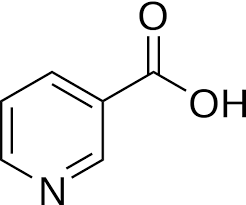
Niacin (also known as vitamin B3, nicotinic acid and vitamin PP) is one of the essential human nutrients. It is a precursor to NAD+/NADH and NADP+/NADPH, which play essential metabolic roles in living cells. Niacin is involved in both DNA repair, and the production of steroid hormones in the adrenal gland.
Niacin deficiency is sometimes seen in developed countries among alcoholics, whose intestine absorption is extremely low, but is especially common in conditions of poverty and malnutrition, in areas where people eat maize as a staple food.
Deficiency of niacin in the diet ( and subsequently lack of NAD-dependent enzymes, especially dehydrogenasis ) causes the disease pellagra, which is characterized by the “three D”: diarrhoea, dermatitis, and dementia, as well as “necklace” lesions on the lower neck, hyper-pigmentation, thickening of the skin, inflammation of the mouth and tongue, digestive disturbances, amnesia, delirium, and eventually death, if left untreated. Common psychiatric symptoms of niacin deficiency include irritability, poor concentration, anxiety, fatigue, restlessness, apathy, and depression.

MELATONIN

Melatonin, also known chemically as N-acetyl-5-methoxytryptamine, is a naturally occurring compound found in animals, plants, and microbes. In animals, circulating levels of the hormone melatonin vary in a daily cycle, thereby allowing the entrainment of the circadian rhythms of several biological functions. Many biological effects of melatonin are produced through activation of melatonin receptors while others are due to its role as a pervasive and powerful antioxidant with a particular role in the protection of nuclear and mitochondrial DNA. Melatonin is secreted into the blood by the pineal gland in the brain. Known as the "hormone of darkness," it is secreted in darkness in both day-active (diurnal) and night-active (nocturnal) animals.
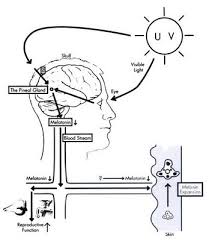
Melatonin has been studied for the treatment of cancer, immune disorders, cardiovascular disease, depression, seasonal affective disorders (SAD), circadian rhythm sleep disorder and sexual dysfunctions.
About melatonin and mood disorders, the cyclic nature of depressive illness, the diurnal variations in its symptomatology and the existence of disturbed sleep-wake and core body temperature rhythms, all suggest that dysfunction of the circadian time keeping system may underlie the pathophysiology of depression. As a rhythm-regulating factor, the study of melatonin in various depressive illnesses has gained attention. Melatonin can be both a ‘state marker’ and a ‘trait marker’ of mood disorders. Measurement of melatonin either in saliva or plasma, or of its main metabolite 6-sulfatoxymelatonin in urine, has documented significant alterations in melatonin secretion in depressive patients during the acute phase of illness.
For a diagnosis of a major depressive disorder at least five of the following symptoms must have been present continuously for more than 2 weeks:
1. depressed mood
2. markedly diminished interest in work
3. significant weight loss
4. insomnia or hypersomnia
5. psychomotor agitation
6. fatigue or loss of energy
7. feelings of worthlessness
8. diminished ability to concentrate
9. recurrent thoughts of death
(from Kahn 1999).
The nature and extent of disruption of melatonin secretion in major depressive disorder has been under intense investigation during the last few decades, ever since Wetterberg and co-workers (1979) formulated the ‘low melatonin syndrome’ hypothesis, the concept that low melatonin secretion can be a biological marker for susceptibility to depressive disorders. Numerous studies have substantiated a deficiency of melatonin secretion in depressives (Brown et al. 1985a,b; Claustrat et al. 1984; Miles and Philbrick 1988; Nair et al. 1984; Paparrigopoulos et al. 2001; Sack and Lewy 1988; Venkoba rao et al. 1983; Zeiten et al. 1987). In some studies clinical symptoms such as suicidal ideas correlated with the decrease in melatonin levels (Venkoba Rao et al. 1983). It has been suggested that the low melatonin levels seen in depressives are due to low norepinephrine and serotonin levels in the brain.
The Therapeutic Potential of Melatonin: A Review of the Science: Brain Function, Neuropsychiatry, and Behavior
SEROTONIN
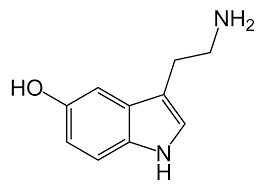
Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Biochemically derived from tryptophan, serotonin is primarily found in the gastrointestinal (GI) tract, platelets, and in the central nervous system (CNS) of animals including humans. It is popularly thought to be a contributor to feelings of well-being and happiness. Approximately 90% of the human body's total serotonin is located in the enterochromaffin cells in the gut, where it is used to regulate intestinal movements. The remainder is synthesized in serotonergic neurons of the CNS where it has various functions. These include the regulation of mood, appetite, and sleep. Serotonin also has some cognitive functions, including memory and learning. Modulation of serotonin at synapses is thought to be a major action of several classes of pharmacological antidepressants. Serotonin secreted from the enterochromaffin cells eventually finds its way out of tissues into the blood. There, it is actively taken up by blood platelets, which store it. When the platelets bind to a clot, they disgorge serotonin, where it serves as a vasoconstrictor and helps to regulate hemostasis and blood clotting. Serotonin is also a growth factor for some types of cells, which may give it a role in wound healing.
Serotonin is mainly metabolized to 5-HIAA, chiefly by the liver: metabolism involves first oxidation by monoamine oxidase ( MAO ) to the corresponding aldehyde, this is followed by oxidation aldehyde dehydrogenise to 5-HIAA, the indole acetic acid derivative. The kidneys then excrete the latter.

Among the chemical neurotransmitter substances, serotonin is perhaps the most implicated in the treatment of various disorders, including anxiety, depression, obsessive-compulsive disorder, schizophrenia, migraine and nausea. The linkage of serotonin to depression has been thoroughly investigated by numerous studies in the last few years (see articles on PubMed about the linkage of serotonin levels to human mood and social interactions:
The role of serotonin in human mood and social interaction. Insight from altered tryptophan levels
Tryptophan, serotonin and human social behavior )
The most concrete evidence of this connection is the decreased concentration of serotonin metabolites like 5-HIAA (5-hydroxyindole acetic acid) in the cerebrospinal fluid and brain tissues of depressed people. If depression, as suggested, is a result of decreased levels of serotonin in the brain, pharmaceutical agents that can reverse this effect should be helpful in treating depressed patients. Therefore, the primary targets of various antidepressant medications are serotonin transports of the brain. Since serotonin is activated when released by neurons into the synapse, antidepressants function at the synapse to enhance serotonin activity. Normally, serotonin actions in the synapse are terminated by its being taken back into the neuron, therefore antidepressants work by blocking serotonin reuptake.
The newest medications used to cure depression are collectively known as selective serotonin inhibitors (SSRIs). These drugs work by altering the function of neurons that release serotonin and blocking the reuptake of serotonin back into the cell. Therefore the level of serotonin activity is increased in any part of the nervous system that uses this neurotransmitter. The SSRIs have became the drug of choice because they have fewer side effects than drugs previously used (especially Monoamine oxidase inhibitors which prevent the breakdown of various monoamine neurotransmitters, including serotonin, and therefore increase concentrations of different neurotransmitters in the brain) due to their limited action to reuptake serotonin alone.
Fluoxetine, commonly known as Prozac, has become so widespread that it is not only used to treat depression, but also used for people that lack self-esteem, fear rejection, and lack the ability to experience pleasure. Unfortunately, although these SSRIs are effective in treating depression and are the least toxic of the antidepressants, they do produce some harmful side effects. In fact, some have defined the presence of certain side effects due to SSRIs as "serotonin syndrome”.
Management of Serotonin Syndrome Reviewed
The serotonin syndrome is generally caused by a combination of two or more drugs, one of which is often a selective serotonergic medication. The drugs which we know most frequently contribute to this condition are the combining of MAOIs with Prozac or other drugs that have a powerful effect upon serotonin. The combination of lithium with these selective serotonergic agents has been implicated in enhancing the serotonin syndrome. The tricyclic antidepressants, lithium, MAOIs, SSRIs, ECT (electric shock treatment) and the serotonin agonists (fenfluramine) all enhance serotonin neurotransmission and can contribute to this syndrome. Anything that will raise the level of serotonin can bring on this hyperserotonergic condition. If the medication is not discontinued the condition can progress rapidly to a more serious state and become fatal. It should be apparent that the greater the enhancement of serotonin levels, the greater the chances of producing the serotonin syndrome.
Because of the side effects of the classical therapies, researchers have been trying to find a safer way to raise serotonin levels: 5-Hydroxytryptophan (5-HTP), an amino acid that is the intermediate step between tryptophan and serotonin, and that unlike the latter can cross the blood-brain barrier, is useful for a wide range of health problems derived from a lack of brain serotonin. Problems such as insomnia, depression, obesity, eating disorders, PMS, panic attacks, alcoholism, anxiety disorders, and bulimia can be successfully treated with 5-HTP as a natural replacement for prescription antidepressants.
According to other researches, an even better way to raise serotonin levels naturally would be to get daily exercise. Studies have shown how muscular work, along with a good diet, can help raise serotonin levels. Such researches may lead to patients learning how to treat themselves without relying on any type of prescription drugs, without experiencing the negative effects these carry with them
HOW TO CONTROL SEROTONIN LEVEL WITHOUT DRUGS
The following factors can cause low serotonin levels:
• Artificial sweeteners (aspartame)
• Caffeine
• Cigarette Smoking
• Diabetes
• Dietary deficiencies of nutrient co-factors
• Ecstasy, Diet Pills, and certain medications
• Genetic Predisposition
• Hormone Imbalances (thyroid, adrenal, estrogen)
• Hypoglycemia
• Insulin Resistance
• Inflammation
• Infections
• Poor Diet
• Lack of exercise
• Lack of sunlight
• Problems converting tryptophan to Serotonin
• Problems with Digestion
• Stress and Anger
• High Cortisol Levels
Serotonin foods help increase serotonin naturally:
• complex carbohydrates
• chicken
• turkey
• tuna
• salmon
• kidney beans
• rolled oats
• lentils
• chickpeas
• pumpkin seeds
• sunflower seeds
• baked potato with skin
• tahini (sesame butter)
• walnuts
• avocado
• almond butter
There are some activities that can elevate your mood and induce a happy feeling. They are:
• Getting exposed to sunlight in the morning will burn melatonin molecules (molecules inducing sleep) and replace them with serotonin.
• Exercising or simply brisk-walking helps to 'feel good'.
• Sleeping well for 5-6 hours or getting early but taking a short nap during the day helps to rejuvenate serotonin levels.
CONCLUSION
Tryptophan and its derivatives perform therefore a fundamental role in various aspects of everyday life, and modulate the relationship of the individual with the environment (regulation of circadian rhythms), and his/her perceptions (appetite, mood) carrying out an invaluable action that grants the execution of everyday activities and the interaction of the individual in the society.
Many efforts are particularly involved in the understanding of its effects on the regulation of mood in order to associate traditional drugs with new complementary and alternative medicine (CAM) techniques for individuals with mood disorders.
Complementary and Alternative Medicine Therapies in Mood Disorders
LINK