DEFINITION
A short protein description with the molecular wheight, isoforms, etc...
Use, when available, the link to Wikipedia (Es Trypsin)

Calcification of joints and arteries
is caused by homozygous or compound heterozygous mutation in the NT5E gene on chromosome 6q14.
Pubmed nt5e mutations and arterial calcifications
NT5E mutations and arterial calcifications including Torino patients
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
It requires Zinc
** PDB
* Quaternary structure
Protein Aminoacids Percentage
The Protein Aminoacids Percentage gives useful information on the local environment and the metabolic status of the cell (starvation, lack of essential AA, hypoxia)
Protein Aminoacids Percentage (Width 700 px)

SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
biological function

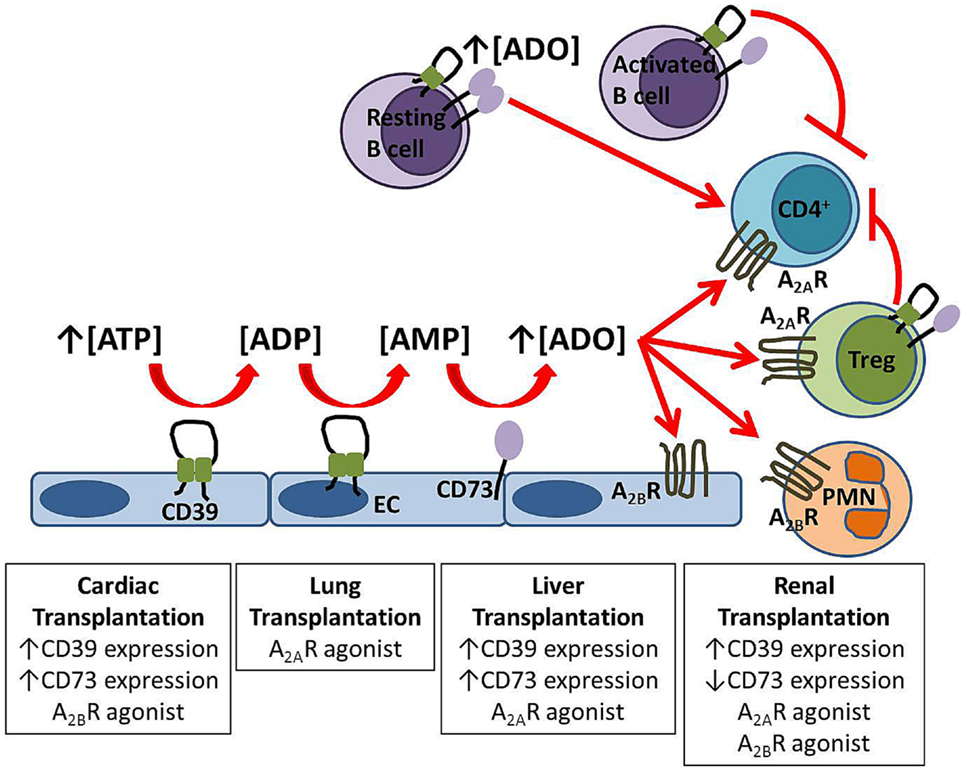
if we have less adenosine what can we expect by a poor stimulation of Adenosine receptors family?
More autoimmunity?
Pbmed NT5E and Treg
Pbmed CD73 and Treg
TH17 and Treg number and interactions are largely dependent on adenosine concentration and therefore can be shifted in the direction of hyperreactivity.

Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. - PubMed - NCBI
Human osteoblast precursors produce extracellular adenosine, which modulates their secretion of IL-6 and osteoprotegerin. - PubMed - NCBI
altre cause di calcificazione
Biology of RANK, RANKL, and osteoprotegerin. 2007
- The discovery of the receptor activator of nuclear factor-kappaB ligand (RANKL)/RANK/osteoprotegerin (OPG) system and its role in the regulation of bone resorption exemplifies how both serendipity and a logic-based approach can identify factors that regulate cell function. Before this discovery in the mid to late 1990s, it had long been recognized that osteoclast formation was regulated by factors expressed by osteoblast/stromal cells, but it had not been anticipated that members of the tumor necrosis factor superfamily of ligands and receptors would be involved or that the factors involved would have extensive functions beyond bone remodeling. RANKL/RANK signaling regulates the formation of multinucleated osteoclasts from their precursors as well as their activation and survival in normal bone remodeling and in a variety of pathologic conditions. OPG protects the skeleton from excessive bone resorption by binding to RANKL and preventing it from binding to its receptor, RANK. Thus, RANKL/OPG ratio is an important determinant of bone mass and skeletal integrity. Genetic studies in mice indicate that RANKL/RANK signaling is also required for lymph node formation and mammary gland lactational hyperplasia, and that OPG also protects arteries from medial calcification. Thus, these tumor necrosis factor superfamily members have important functions outside bone. Although our understanding of the mechanisms whereby they regulate osteoclast formation has advanced rapidly during the past 10 years, many questions remain about their roles in health and disease. Here we review our current understanding of the role of the RANKL/RANK/OPG system in bone and other tissues.
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
1α,25-dihydroxyvitamin D3 acts via transforming growth factor-β to up-regulate expression of immunosuppressive CD73 on human CD4+ Foxp3- T cells. 2015
Abstract
Vitamin D deficiency is associated with increased incidence and severity of various immune-mediated diseases. Active vitamin D (1α,25-dihydroxyvitamin D3; 1,25(OH)2 D3) up-regulates CD4 T-cell expression of the purine ectonucleotidase CD39, a molecule that is associated with the generation of anti-inflammatory adenosine. Here we aimed to investigate the direct impact of 1,25(OH)2 D3 on expression of the downstream ecto-5-nucleotidase CD73 by human CD4 T cells, and components of the transforming growth factor-β (TGF-β) pathway, which have been implicated in the modulation of CD73 by murine T cells. At 10(-8) to 10(-7) m, 1,25(OH)2 D3 significantly increased expression of CD73 on peripheral human CD4 T cells. Although 1,25(OH)2 D3 did not affect the mRNA expression of latent TGF-β1 , 1,25(OH)2 D3 did up-regulate expression of TGF-β-associated molecules [latency-associated peptide (LAP), glycophorin A repetitions predominant (GARP), GP96, neuropilin-1, thrombospondin-1 and αv integrin] which is likely to have contributed to the observed enhancement in TGF-β bioactivity. CD73 was highly co-expressed with LAP and GARP following 1,25(OH)2 D3 treatment, but unexpectedly, each of these cell surface molecules was expressed primarily on CD4 Foxp3 T cells, rather than CD4 Foxp3 T cells. Notably, neutralization of TGF-β significantly impaired 1,25(OH)2 D3-mediated induction of CD73. Collectively, we show that 1,25(OH)2 D3 enhances expression of CD73 on CD4 Foxp3 T cells in a process that is at least partially TGF-β-dependent. These data reveal an additional contributing mechanism by which vitamin D may be protective in immune-mediated disease.
DIAGNOSTIC USE
Adenosine receptor signaling: a key to opening the blood-brain door. 2015
Collectively, these findings point to AR manipulation as a pertinent avenue of research for novel strategies aiming at efficiently delivering therapeutic drugs/cells into the CNS, or at restricting the entry of inflammatory immune cells into the brain in some diseases such as multiple sclerosis. CD73