CRUCIFERAE
Biolè Carloalberto - Basile Nicola
DESCRIPTION

CRUCIFERAE , of the order Brassicales, are a large assemblage of mostly herbaceous plants. The family includes many plants altered and domesticated by humans. They are called Cruciferae because their members’ flowers are in the form of a Greek cross, with four petals.
The most important genus is Brassica, including the cabbages, mustards, and rapes. One species, B. oleracea, has many edible varieties, such as broccoli, Brussels sprouts, cabbage, cauliflower, kale.
In the last years several studies have been done to find some relations between Cruciferous intake and cancer protection. The molecules responsible of these effects came out to be isothiocyanate and their metabolites.
THE MOLECULES
The active molecules are Indole-3-carbinol (1H-indol-3-ylmethanol IUPAC name) and isothiocyanates (mostly sulforaphane: 1-Isothiocyanato-4-methylsulfinylbutane).
Indole-3-carbinol has a indole with a hydroxymethyl group that represents the hydrophilic group.
 Sulfurofane is a isothiocyanate, it means that it has a –N=C=S chemical group, formed by substituting sulfur for oxygen in the isocyanate group, bounded to a big alkyl chain containing a sulfinyl S=O group.
Sulfurofane is a isothiocyanate, it means that it has a –N=C=S chemical group, formed by substituting sulfur for oxygen in the isocyanate group, bounded to a big alkyl chain containing a sulfinyl S=O group.
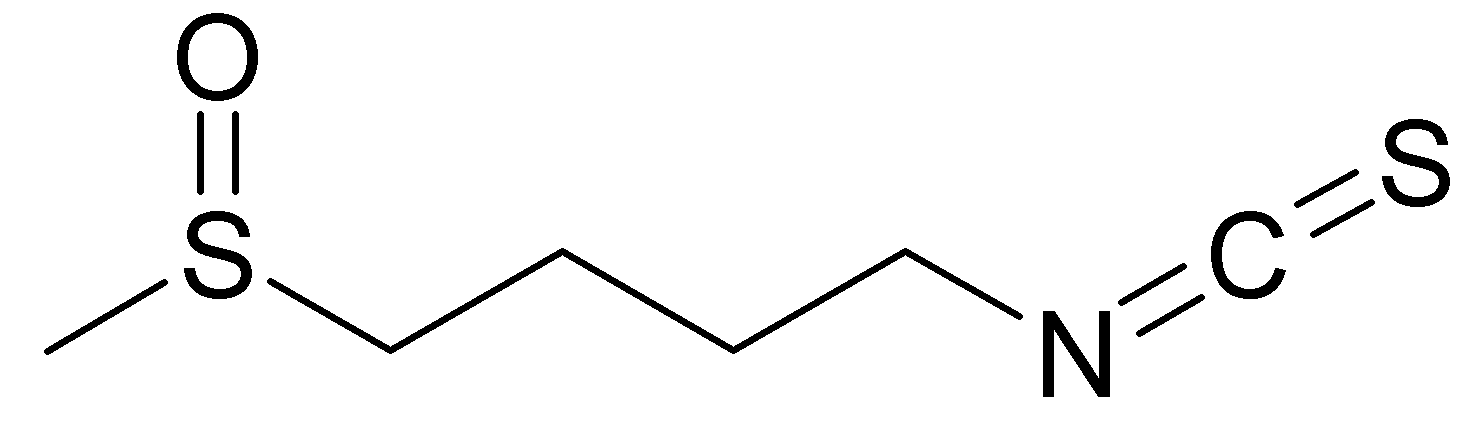
Both indole-3-carbinol and sulphoraphane derive from glucosinolates. Glucosinolates are a class of organic compounds that contain sulfur and nitrogen and are derived from glucose and an amino acid. They occur as secondary metabolites of almost all plants of the order Brassicales.
GENERAL EFFECTS ON HEALTH
I3C has been shown to have a chemopreventive action on several human cancers. The first and greatest effects concern breast and cervical estrogen-dependant cancer. Later, many researches managed to relate I3C with the prevention from colon, lung and prostate cancer too.
Given that, I3C can be considered a general protector against many kinds of neoplasy. At the same time other groups found that it could also play a role in improving Systemic Lupus Erythematosus patient conditions.
The micronutrient indole-3-carbinol: implications for disease and chemoprevention, 2000
Sulforaphane, like I3C, is useful against many types of cancer. Moreover, it has antimicrobial properties, as it appears to help eradicate Helicobacter Pylori from the stomach.
Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention, 2010
PHARMACOKINETICS
There is no Indole-3-carbinol in Brassicales. There are only glucobrassicin and an enzyme called myrosinase. The leading role of cruciferae effects on human health is played by the enzyme Myrosinase. This is an enzyme naturally found in plants involved in defense against herbivores. It can hydrolyze glucosinolates giving origin to compounds that have different features, from toxic (anti-herbivores) to salutary ones. Thanks to it Indole-3 carbinol and sulforaphane are produced.
The enzyme and its substrates are separated one from the other. When cooking or chewing we break the cells and let substances react.
When myrosinase and glucobrassicin meet indole-3-carbinol is formed, via Indole-3-methyl isothiocianate. This molecule is unstable and loses his -SCN group originating I3C. When swallowing I3C enters the stomach and is exposed to its acidic environment.
There, I3C molecules can combine with each other to form a mixture of biologically active compounds, known as acid condensation products.
Among the others the most important are the dimer 3,3'-diindolylmethane (DIM) and a cyclic trimer (CT). It is thought that the health benefits examined of I3C are not only dued to I3C itself but also to a myriad of other metabolites, some of them still unknown to us. Recently there has been a great research on the effects of DIM giving great results.
Indole-3-carbinol is produced by the hydrolisis of glucobrassicin. Glucobrassicin is a glucosinolate compound found in high concentration in Cruciferous plant family.
Similarily, the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane.
Both Indole-3-methyl isothiocianate (I3C unstable precursor) and sulforaphan are isothiocyanate, while I3C is not.
For what concerns bioavailability, these beneficial compounds can be found in Brassicaceae. Although glucosinolates are present in relatively high concentrations in cruciferous vegetables, glucobrassicin makes up only about 8-12% of the total glucosinolates. The amount of indole-3-carbinol formed from glucobrassicin in foods is variable and depends, in part, on the processing and preparation of foods.
Glucosinolates are water-soluble and can be leached into cooking water. Boiling from 9-15 minutes resulted in 18-59% decrease in the total glucosinolate content of cruciferous vegetables. Steaming or microwaving, may reduce glucosinolate losses. However boiling, steaming, and microwaving at high power (850-900 watts), may inactivate myrosinase. Even in the absence of plant enzymatic activity, the myrosinase activity of human intestinal bacteria results in some glucosinolate hydrolysis. However, studies in humans have found that inactivation of myrosinase in cruciferous vegetables substantially decreases the bioavailability of glucosinolate hydrolysis products. Furthermore, I3C and DIM extracts and supplements containing extracts of cruciferous vegetables are commercially available.
I3C, in addition to its acid condensation products, is absorbed from the gut and distributed systemically into a number of well-perfused tissues, thus allowing the possibility for some pharmacological activity of the parent compound in vivo.
I3C is rapidly absorbed, distributed, and eliminated from plasma and tissues, falling below the limit of detection by 1 h . Highest concentrations of I3C can be detected in the liver where levels are approximately 6-fold higher than those in the plasma. Levels of DIM and other acid condensation products are much lower, but stay in plasma and tissues longer.
Pharmacokinetics and Tissue Disposition of Indole-3-carbinol and Its Acid Condensation Products after Oral Administration to Mice, 2004
Sulforaphane is very well and rapidly absorbed and displays an absolute bioavailability of 82 %, which, however, decreases at the higher doses, indicating a dose-dependent pharmacokinetic behaviour; at high dose, the rate of absorption constant kab and biological half-life decrease significantly. Sulforaphane is rapidly absorbed, achieving high absolute bioavailability at low dietary doses, but dose-dependent pharmacokinetics is evident, with bioavailability decreasing with increasing dose. It's important to add that SFN decreases markedly in the first 2 hours after intake, and then keeps a low prolonged concentration, that could justify long-term effects.
Absolute bioavailability and dose-dependent pharmacokinetic behaviour of
dietary doses of the chemopreventive isothiocyanate sulforaphane in rat, 2007
HOW DO THEY WORK? PROTECTION FROM CANCER
Many mechanisms have been suggested to explain I3C and sulforaphane effects on health.
I3C has been first investigated as an inhibitor of breast and cervical estrogen-dependent cancer. It does that by interfering with estrogens and estrogen receptor metabolism.
Endogenous estrogens exert their effects by binding to estrogen receptors (ERs). Within the nucleus, the estrogen-ER complex can bind to DNA sequences in genes known as estrogen response elements (EREs). Some ER-mediated effects promote cellular proliferation in the breast and uterus.
I3C can interfere with estrogen metabolism in many ways.
First of all, I3C can inhibit the transcription of estrogen-responsive genes stimulated by 17beta-estradiol. Acid condensation products of I3C may also inhibit the transcription of estrogen-responsive genes by competing for coactivators or increasing ER degradation. Estrogen-responsive breast cancer cells express both estrogen receptor (ER)-alpha (ERalpha) and ERbeta. Indole-3-carbinol (I3C) strongly down-regulates ERalpha protein and transcript levels, without altering the level of ERbeta protein. I3C disrupts17beta-estradiol stimulation of estrogen response element (ERE) activity as well as of endogenous progesterone receptor transcripts. ERbeta is activated in indole-treated cells. A significantly higher ERbeta:ERalpha ratio is generally highly associated with antiproliferative status of human breast cancer cells.
Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells, 2006
The estrogen 17beta-estradiol can be irreversibly metabolized to 16alpha-hydroxyestrone (16OHE1) or 2-hydroxyestrone.
In contrast to 2OHE1, 16OHE1 is highly estrogenic and can stimulate the proliferation of several estrogen-sensitive cancer cell lines. It is thought that shifting the metabolism of 17beta-estradiol toward 2OHE1, and away from 16OHE1, could decrease the risk of estrogen-sensitive cancers, such as breast cancer. Moreover estradiol increases expression of HPV oncogenes responsible for cervical cancer, whereas I3C and the estrogen metabolite 2-hydroxyestrone (2-OHE) abrogate the estrogen-increased expression of HPV oncogenes. I3C enhances gene expression of CYP enzymes responsible for 2-hydroxylation of estrogen, and induces the formation of 2-OHE.
2-hydroxyestrone: the 'good' estrogen, 1996
Induction by estrogen metabolite 16 alpha-hydroxyestrone of genotoxic damage and aberrant proliferation in mouse mammary epithelial cells, 1992
Anti-estrogenic activities of indole-3-carbinol in cervical cells: implication for prevention of cervical cancer, 1999
Abrogation of estrogen-mediated cellular and biochemical effects by indole-3-carbinol, 2001
The other main mechanism of action is the induction of detoxification enzymes and activation of the antioxidant, electron scavenger, apparatus of the cell.
I3C and sulforaphane protect from cancer inducing the expression of Nrf2, NADH quinine oxidoreductase 1 (NQO-1) and apoptosis related biomarkers. They induce Nrf2-mediated genes, one of them being antioxidant response element (ARE). NF-E2-related factor 2 (Nrf2) is a transcription factor that binds to the promoter sequence antioxidant responsive element leading to coordinated up-regulation of ARE-driven detoxification and antioxidant genes.
In vivo pharmacodynamics of indole-3-carbinol in the inhibition of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: Involvement of Nrf2 and cell cycle/apoptosis signaling pathways, 2011
Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates, 2011
Likewise, sulforaphane, I3C and his acid condensation products can bind to aryl hydrocarbon receptor (AhR). Binding allows the AhR to enter the nucleus to form a complex with the Ahr nuclear translocator (Arnt) protein. This complex enhances transcription of xenobiotic response elements (XRE) including CYP enzymes and several phase II enzymes. Thus, the activity of phase I and phase II enzymes are increased. This is generally considered a beneficial effect because the elimination of potential carcinogens or toxins is enhanced. However, some carcinogens require transformation by phase I enzymes to become active carcinogens, and this may be a side effect.
Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines, 2001
The synergistic upregulation of phase II detoxification enzymes by glucosinolate breakdown products in cruciferous vegetables, 2001
Induction of rat pancreatic glutathione S-transferase and quinone reductase activities by a mixture of glucosinolate breakdown derivatives found in Brussels sprouts, 1998
Furthermore, many papers have been published looking into new pathways in which I3C may be involved; however, these ones are not fully understood.
"I3C inhibits prostate cancer androgen receptor mediated progression through suppressing EGF (dependent or independent)-induced cell migration through beta-catenin degradation.
Indole-3-carbinol inhibits prostate cancer cell migration via degradation of beta-catenin, 2011
I3C protects from cancer through inhibition of Akt activity and p53-independent p21 and GADD45A overexpression by his metabolite CT.
The indole-3-carbinol cyclic tetrameric derivative CTet inhibits cell proliferation via overexpression of p21/CDKN1A in both estrogen receptor-positive and triple-negative breast cancer cell lines, 2011
Indole-3-carbinol induces apoptosis through p53 and activation of caspase-8 pathway in lung cancer A549 cells, 2010
Sulforaphane, similarily, is thought to act on apoptosis induction and cell cycle regulation.
Cell death induction by isothiocyanates and their underlying molecular mechanisms, 2006.
Vegetable-derived isothiocyanates: anti-proliferative activity and mechanism of action, 2006)
Furthermore, sulforaphane is an antiinflammatory compound
Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms
HOW DO THEY WORK? I3C AND LUPUS, SULFORAPHANE AND H.PYLORI
After discovering the great potential of I3C in oncology, scientists observed an improvement of conditions in patients affected by Systemic Lupus Eritematous. This can be explained with an increased urinary 2OHE1:16OHE1 ratio (estrogen is thought to play a role in SLE because it is much more common in women than men, and its onset is most common during the reproductive years).
Lifespan is prolonged in autoimmune-prone (NZB/NZW) F1 mice fed a diet supplemented with indole-3-carbinol, 2003
Indole-3-carbinol in women with SLE: effect on estrogen metabolism and disease activity, 2001
It is almost certain now that sulforaphane has an antimicrobial other than the cancer preventing now, being almost certanly inhibitor of H. Pylori.
Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli ( Brassica oleracea L.) sprouts, 2010
SIDE EFFECTS
An important side effect of an big intake of glucosinolates and isothiocyanates from Cruciferae is goitre. It is determined by the inhibition of iodine uptake in the thyroid. In fact, the Na+/I- symporter, also called NIS or SCL5a, is inhibited by SCN- thiocyanate group.
Different electrophysiological character of I-, ClO4-, and SCN- in the transport by Na+/I- symporter, 1997.
Bioactive organosulfur phytochemicals in Brassica oleracea vegetables--a review, 1995
The role of goitrogenic factors distinct from iodine deficiency in the etiology of goiter, 1988
An excessive intake of I3C (for example because of a supplement) may have side effects because of the alterated estrogen balance; a problem could be for example osteoporosis because of the alteration of estrogen metabolism, similar to a menopausal effect.
Dietary and pharmacological control of estradiol metabolism in humans, 1990
We mentioned I3C can increase enzymatic activity, in particular CYP1A1 and CYP1A2. This is important for its xenobiotic activity, but it may have collateral effects interacting with drugs, toxic substances, endogenous metabolites;
KEGG CYP1A1
KEGG CYP1A2
Evaluation of chronic dietary exposure to indole-3-carbinol and absorption-enhanced 3,3'-diindolylmethane in sprague-dawley rats, 2003