DESCRIPTION
Aspirin, also known as acetylsalicylic acid (abbreviated ASA), is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. Aspirin also has an antiplatelet effect by inhibiting the production of thromboxane, which under normal circumstances binds platelet molecules together to create a patch over damaged walls of blood vessels.
It is the salicylate ester of acetic acid; in fact, its synthesis is classified as an esterification reaction:

Salicylic acid is treated with acetic anhydride causing a chemical reaction that turns salicylic acid's hydroxyl group into an ester group (R-OH → R-OCOCH3). This process yields aspirin and acetic acid. Small amounts of sulfuric acid (and occasionally phosphoric acid) are almost always used as a catalyst.
A little bit of history
Plant extracts, including willow bark and spiraea, of which salicylic acid was the active ingredient, had been known to help alleviate headaches, pains, and fevers since antiquity. The father of modern medicine, Hippocrates, left historical records describing the use of powder made from the bark and leaves of the willow tree to help these symptoms.
A French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid in 1853.
In 1897, chemists working at Bayer AG produced a synthetically altered version of salicin, derived from the species Filipendula ulmaria, which caused less digestive upset than pure salicylic acid. The identity of the lead chemist on this project is a matter of controversy. Bayer states the work was done by Felix Hoffmann, but the Jewish chemist Arthur Eichengrün later claimed he was the lead investigator and records of his contribution were expunged under the Nazi regime. The new drug, formally acetylsalicylic acid, was named Aspirin, derived from "acetyl" and "Spirsäure", an old German name for salicylic acid.
The popularity of aspirin grew over the first half of the 20th century, spurred by its supposed effectiveness in the wake of the Spanish flu pandemic of 1918. (However, recent research suggests the high death toll of the 1918 flu was partly due to aspirin, as the doses used at times can lead to toxicity, fluid in the lungs, and, in some cases, contribute to secondary bacterial infections and mortality.)
Today, aspirin is one of the most widely used medications in the world, with an estimated 40,000 tonnes of it being consumed each year.

CLASSIFICATION
- Cyclooxygenase inhibitors
- Antimigraines
- Antithrombosis agents
- Benzoic acid family
INDICATIONS
- Anti-inflammatory activity:
Aspirin is part of a group of medications called NonSteroidal Anti-Inflammatory Drugs (NSAIDs): its anti-inflammatory effect is performed by inhibiting cyclooxygenase2 or COX2 (prostaglandin synthetase2) thereby reducing the synthesis of prostaglandins and thromboxanes, which are the main mediators of inflammation.
It is indicated for the relief of the signs and symptoms of inflammatory diseases such as rheumatoid arthritis, juvenile rheumatoid arthritis, osteoarthritis and arthritis.
- Analgesic effect:
By inhibiting COX2 aspirin can also relieve pain, because it blocks the synthesis of algesic prostaglandins.
It is particularly indicated for headache and migraine.
- Antipyretic effect:
Like its ability to control pain, aspirin's ability to control fever is due to its action on the prostaglandin system through its irreversible inhibition of COX, particularly COX2.
- Effects on platelets:
By inhibiting COX1 indeed aspirin reduces platelet aggregation: infact it reduces platelet production of TXA2 (thromboxane - powerful vasoconstrictor that lowers cyclic AMP and stimulates blood platelet aggregation, essential to the role of platelets in blood clotting) by COX1. Most cells can synthesize new cyclooxygenase, but platelets cannot, because they lack a nucleus. Therefore, aspirin causes an irreversible effect on platelet aggregation.
Many people take a daily aspirin for its anti-clotting effect, attributed to inhibition of thromboxane formation in blood platelets.
PHARMACOKINETICS
Absorption Aspirin is a weak acid (pka=3,4), so in the acidic conditions of the stomach (pH=1,4) using the Henderson-Hasselbach equation we obtain:
[A-]/[AH] = 10(pH - pKa) = 10(1,4 - 3,4) = 10^-2 = 1/100
Very little of it is ionized in the stomach after oral administration, so the undissociated form (more lipophilic and easier to absorb) prevails. Theoretically, these weakly acidic drugs like aspirin are more readily absorbed from an acid medium (stomach) than from a basic one (intestine), where we can have a pH=8,4:
[A-]/[AH] = 10(pH - pKa) = 10(8,4 - 3,4) = 10^5 = 100000
Aspirin can be considered completely ionized.
However, aspirin and couple other weak organic acids don't follow normal kinetics across lipid membranes: in fact, most absorption occurs in the small intestine, because the surface area is larger and membranes are more permeable (the general explanation has to do with micoenvironments at the surface of the enterocytes, where the pH only there favors the unionized form (HA) long enough that it is absorbed across the membrane).
Aspirin is very rapidly absorbed from the gastrointestinal tract when administered as a solution, and somewhat more slowly when administered in tablets. The rate of absorption is dependent upon factors as stomach content, gastric emptying times, tablet disintegration rates and gastric pH.
Following absorption, aspirin is hydrolized to salicycic acid with peak plasma levels of salicylic acid occurring within 1-2 hours of dosing. (In plasma, where pH=7,4, we can consider salicylic acid completely ionized, in the salicylate form).
Distribution Salicylate distributes widely to all tissues and fluids in the body including the central nervous system (CNS). The highest concentrations are found in the plasma, liver, renal cortex, heart and lungs. At low concentrations (<100 micrograms/mL), approximately 90 percent of plasma sacylate is buond to albumin while at higher concentrations (>400 micrograms/mL), only about 75 percent is bound.
It's important to note that salicylate can be found in breast milk and may cross the placental barrier and distributes into fetal tissues.
Metabolism As much as 80% of therapeutic doses of salicylate is metabolized in the liver. This metabolism occurs primarily by hepatic conjugation with glycin to form salicyluric acid or with glucuronic acid to form salicyl acyl and phenolic glucuronide, involving different metabolic pathways. Minor metabolites formed include gentisic acid, which appears to be the only active metabolite, but because of its small concentrations, it appears to play an insignificant role therapeutically.
The predominant pathway is the conjugation with glycin, which is saturable. With low doses of aspirin approximately 90% of salicylate is metabolized through this pathway. As the maximum capacity of this major pathway is reached, the other pathways with a lower clearance become more important. Therefore, the half-life of salicylate depends on the major metabolic pathway used at a given concentration and becomes longer with increasing dosage.
Elimination Salicylates are excreted mainly by the kidneys as salicyluric acid (75%). Urinary excretion of free salicylate accounts for 10% of the total elimination of salicylate. When small doses (less than 250 mg in an adult) are ingested, all pathways proceed by first-order kinetics, with an elimination half-life of about 2.0 to 4.5 hours. When higher doses of salicylate are ingested (more than 4 g), the half-life becomes much longer (15–30 hours), because the biotransformation pathways concerned with the formation of salicyluric acid and salicyl phenolic glucuronide become saturated. Renal excretion of salicylic acid becomes increasingly important as the metabolic pathways become saturated, because it is extremely sensitive to changes in urinary pH: as the urinary pH rises from 5 to 8, the amount of free ionized salicylate excreted increases from 3% of the total salicylate dose to more than 80% (by ion trapping in the urine).
Salicylate pharmacokinetic parameters
| Clearance (CL) | 3.6 L/h (may decrease to 0.6 L/h depending on dose) |
| Volume of distribution (Vd) | 11.9 L |
| Half-life (t1/2) | 2h (may increase to 30h depending on dose) |
Clearance: rate of drug elimination divided by plasma concentration, giving a volume of plasma from which drug is completely removed per unit of time.
Volume of distribution: fluid volume that would be required to contain the amount of drug present in the body at the same concentration as in the plasma.
Half-life: time it takes for the plasma concentration or the amount of drug in the body to be reduced by 50%.
MOLECULAR MECHANISM
Aspirin belongs to rhe group of NSAIDs, but differs from most other NSAIDs in the mechanism of action.
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (PTGS) enzyme required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the PTGS enzyme, preventing arachidonate binding. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.

(See also COX and drugs)
PHARMACOGENOMICS
Pharmacogenomics is the branch of pharmacology which deals with the influence of genetic variation on drug response in patients by correlating gene expression or single-nucleotide polymorphisms with a drug's efficacy or toxicity.
In particular, I'd like to focus on aspirin as a chemoterapic: regular use of aspirin after diagnosis was associated with longer survival among patients with mutated-PIK3CA colorectal cancer.
Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. 2012
Colorectal cancers (CRCs) are a heterogeneous group of diseases, so there are differences in response to treatment between different colorectal tumors. In West Countries is the third cause of death after breast cancer and lung cancer. These are some statistical datas: in 2008 more than 140000 people in the US were diagnosed with CRC, and about 50000 of people died from CRC. In Italy there were about 50000 of new cases in 2010.
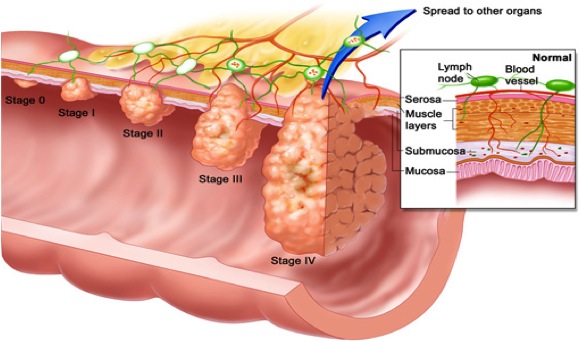
(See also: Red Meat and Colorectal Cancer and Calcium and Colorectal Cancer)
The effect of postdiagnosis aspirin use on survival appears to differ according to tumor expression of PTGS2 (HGNC:9605, the official symbol for prostaglandin-endoperoxide synthase 2, also known as cyclooxygenase-2 or COX-2). In particular, the same authors of this article saw that aspirin had a major effect on cells that overexpress PTGS2. But it’s hard to think to PTGS2 as a new biomarker because the standardization of the PTGS2 immunohistochemical assay is very challenging. So the group purposed to study the correlation between the effect of postdiagnosis use of aspirin and the mutation in PI3KCA (the gene encoding phosphatidylinositol-4,5-bisphosphonate 3-kinase, catalytic subunit alpha polypeptide).
The phosphatidylinositol 3-kinase (PI3K) signaling pathway plays an important role in carcinogenesis (Pathways in cancer). Mutations in PIK3CA are present in approximately 15 to 20% of colorectal cancers.
Up-regulation of PI3K enhances PTGS2 activity and prostaglandin E2 synthesis, resulting in inhibition of apoptosis in colon-cancer cells.
In this study they hypothesized that aspirin may suppress cancer-cell growth and induce apoptosis by blocking the PI3K pathway.
They used data from two prospective cohort studies, the Nurses Health Study that includes about one hundred and twenty thousand of women enrolled in 1976, and the Health Professionals Follow-up Study that includes about fifty thousand of men enrolled in 1986. Every 2 years they have to respond to a questionnaire in which they have to update information about lifestyle factors, new cancers or other diseases, use of drugs and other stuff. Then they extracted DNA from colorectal cancer tissues of CRC patients that was stored in hospitals, sequenced some exons of PI3KCA and of other oncogenes or tumor suppressor genes, and analyzed the expression of PTGS2 too. Among these patients, they found information about tumor tissue, aspirin use, survive and PI3KCA mutation in 964 CRC patients, so they used this subgroup to study the effect of aspirin on survival.
So they tested the hypothesis that the effect of postdiagnosis use of aspirin on survival might be stronger in mutated-PI3KCA CRCs than in wt-PI3KCA cancer. First of all they saw that among patients with mutated PI3KCA there was longer cancer-specific survival among regular users of aspirin post diagnosis; in contrast among patients with the wt gene there wasn’t a cancer specific survival between regular users of aspirin.
Figure 1. Mortality among Patients with Colorectal Cancer, According to Regular Use or Nonuse of Aspirin after Diagnosis and PIK3CA Mutation Status.
Figure 1

Panels A and B show colorectal cancer–specific mortality among patients with mutant-PIK3CA tumors and those with wild-type PIK3CA tumors, respectively, and Panels C and D show overall mortality in the respective subgroups of patients.
In particular they saw that in patients with the mutated gene only 3% died in 5 years after the diagnosis using aspirin, whereas 26% died between aspirin non users. Instead we cannot see differences between users or nonusers of aspirin in wild-type PI3KCA patients (15% and 15%).
Table 3. Colorectal Cancer–Specific Survival at 5 Years, According to Tumor PIK3CA Mutation Status and Use or Nonuse of Aspirin after Diagnosis.
Table 3

Then they performed another analysis to determine if the use of aspirin before the diagnosis might have modified the association between aspirin post diagnosis, PI3KCA mutation and survival.
They saw that among mutant PI3K patients that used aspirin post diagnosis there was a reduction in mortality irrespective of aspirin use before the diagnosis.
This result is very important because we can now considerate the PI3KCA mutation as a tumor biomarker (see also Prognostic and Predictive Biomarkers in Resected Colon Cancer) that could predict the response to the initiation of aspirin therapy in patients with newly diagnosed CRC, so we are in the field of the personalized medicine.
Boris Pasche, PhD of the University of Alabama, said this sentence that perfectly expresses the results of this study: "aspirin may will become one of the oldest drugs to be used as a 21st century targeted therapy".
SIDE EFFECTS
- Reye's syndrome:
Reye's syndrome, a rare but severe illness characterized by acute encephalopathy and fatty liver, can occur when children or adolescents are given aspirin for a fever or other illnesses or infections.
- Tinnitus (acuphenes):
Tinnitus, from the Latin word tinnītus meaning "ringing", is the perception of sound within the human ear in the absence of corresponding external sound.
- Gastrointestinal effect: gastric ulceration/bleeding. The gastric mucosa protects itself from gastric acid with a layer of mucus, the secretion of which is stimulated by certain prostaglandins. NSAIDs block the function of cyclooxygenase 1 (cox-1), which is essential for the production of these prostaglandins.
NB:the incidence of duodenal ulcers has dropped significantly during the last 30 years, while the incidence of gastric ulcers has shown a small increase, mainly caused by the widespread use of NSAIDs.
- Coagulating abnormalities: even low doses of aspirin can inhibit platelet function leading to an increase in bleeding time. This can adversely affect patients with inherited (hemophilia) or acquired (liver disease or vitamin K deficiency) bleeding disorders.
TOXICITY
- Aspirin reduces experimental cerebral blood flow in vivo. 1999 Neurol Res. 1999 Jul;21(5):488-90. Bednar MM, Gross CE.
Aspirin therapy for stroke prophylaxis in low risk patients has paradoxically demonstrated an increased risk of ischemic stroke in several studies. Moreover, the MAST-Italy trial reported a near doubling of mortality with the addition of aspirin to thrombolytics while experimentally, we have noted that aspirin antagonizes t-PA-mediated clot lysis. The mechanisms responsible for these observations is unclear. Of interest, few studies have examined the effect of aspirin on cerebral blood flow (CBF). The objective of this study was to examine the acute effect of high dose aspirin on CBF in a rabbit model. Mean arterial pressure, arterial blood gases, and core and brain temperature were controlled throughout the protocol. CBF, measured by the technique of hydrogen clearance using Platinum-Iridium flow probes, was measured before and 20 min following aspirin administration (20 mg kg-1 i.v.) in a cohort of 50 rabbits and compared to rabbits receiving vehicle (n = 19). Following aspirin therapy, CBF (cc/100 g-1 min-1) was reduced from 80.8 +/- 27.4 to 65.1 +/- 31.7 (mean +/- SD), a reduction to 80.4 +/- 21.3% of baseline (p < 0.00001, t-test), whereas CBF in the control group remained unchanged (81.0 +/- 25.4 vs. 77.5 +/- 24.0, mean +/- SD). Thus aspirin acutely reduced CBF by approximately 20% in a rabbit model, perhaps related to inhibitory effects on prostacyclin and/or nitric oxide. This result may help explain the possible increase in ischemic stroke seen in low risk patients on aspirin therapy. A reduction in CBF by aspirin may also assist in understanding the antagonism of t-PA-mediated clot lysis by aspirin seen in our rabbit model of thromboembolic stroke, particularly since all agents which share the ability to reverse this antagonism (nitric oxide donors, beta blockers, hydralazine, prostacyclin) also increase CBF by approximately 20%. Future strategies for 'antiplatelet' therapy may benefit from using agents which do not adversely affect CBF.
RESISTANCE