Targets of curcumin
p=. 
Abstract
Curcumin (diferuloylmethane), an orange-yellow component of turmeric or curry powder, is a
polyphenol natural product isolated from the rhizome of the plant Curcuma longa. For centuries,
curcumin has been used in some medicinal preparation or used as a food-coloring agent. In recent years, extensive in vitro and in vivo studies suggested curcumin has anticancer, antiviral,
antiarthritic, anti-amyloid, antioxidant, and anti-inflammatory properties. The underlying
mechanisms of these effects are diverse and appear to involve the regulation of various molecular targets, including transcription factors (such as nuclear factor-κB), growth factors (such as vascular endothelial cell growth factor), inflammatory cytokines (such as tumor necrosis factor, interleukin 1 and interleukin 6), protein kinases (such as mammalian target of rapamycin, mitogen-activated protein kinases, and Akt) and other enzymes (such as cyclooxygenase 2 and 5 lipoxygenase). Thus, due to its efficacy and regulation of multiple targets, as well as its safety for human use, curcumin has received considerable interest as a potential therapeutic agent for the prevention and/or treatment of various malignant diseases, arthritis, allergies, Alzheimer’s disease, and other inflammatory illnesses. This review summarizes various in vitro and in vivo pharmacological aspects of curcumin as well as the underlying action mechanisms. The recently identified molecular targets and signaling pathways modulated by curcumin are also discussed here.
Introduction
Turmeric(the common name for Curcuma Longa, known as haldi in Hindi) is an Indian spice that belongs to the ginger family.
Besides the use as a spice, food preservative and coloring agent, turmeric has been traditionally used in Ayurvedic medicine for the treatment of various ailments such as arthritis, ulcers, jaundice, wounds, fever, trauma as well as skin diseases like psoriasis. Curcumin, a hydrophobic polyphenol, is a principal active constituent of turmeric. In addition to curcumin, turmeric also contains other constituents termed curcuminoids. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, and the recently identified cyclocurcumin are the major curcuminoids isolated from turmeric .Several studies have shown that curcumin is more active than demethoxycurcumin or bisdemethoxycurcumin. Commercial available preparations of “curcumin” contain approximately 77% curcumin, 17% demethoxycurcumin and 3% bisdemethoxycurcumin.
Curcumin was first isolated in impure form in 1815 by Vogel and Pelletier, and its chemical
structure and synthesis was confirmed by Lampe et al. in 1910 and 1913, respectively.
Chemically, curcumin is a bis-α,β-unsaturated β-diketone, named (E, E)-1,7-bis (4-
hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5 dione

The first study on the use of curcumin in human diseases was published in 1937. Its antibacterial effect and the ability to decrease blood sugar levels in human subjects was documented in 1949 and 1972, respectively. Over the last 60 years, more than 3000 studies have demonstrated curcumin has antioxidant, antibacterial, antifungal, antiviral, anti-inflammatory, antiproliferative, pro- apoptotic and anti-atherosclerotic effects, exerting medicinal benefits against neurodegenerative diseases, arthritis, allergy, inflammatory bowel disease, nephrotoxicity, AIDS, psoriasis, diabetes, multiple sclerosis, cardiovascular disease, and lung fibrosis.

These effects of curcumin have attracted considerable interests of researchers to uncover its multiple cellular targets and the molecular mechanisms underlying these biological properties. Evidence indicates that the pleiotropic effects of curcumin are dependent on its capacity of interacting and regulation of multiple molecular targets.
These targets include transcription factors, growth factors, kinases, inflammatory
cytokines, adhesion molecules, apoptosis-related proteins and others. Herein, we present a
brief review summarizing the recently identified molecular targets and signaling pathways
modulated by curcumin. Various in vitro and in vivo pharmacological aspects of curcumin
as well as the underlying action mechanisms are also discussed here. Because of space
limitation, we apologize for not being able to cite all related published studies. The readers
who are particularly interested in learning more on curcumin as a therapeutic agent are
referred to the excellent review articles.
Molecular targets of curcumin
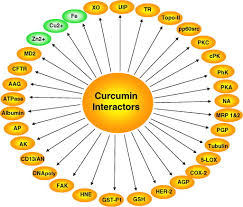
Transcription factors
The transcription factors affected by curcumin might be activated or inhibited depending on
the particular target. Curcumin potently inhibits the activation of some transcription factors including nuclear factor-κB (NF-κB), activated protein-1 (AP-1), signal transducer and activator of transcription (STAT) proteins, hypoxia-inducible factor-1 (HIF-1), Notch-1, early growth response-1 (Egr-1) and β-catenin, but activates other transcription factors such as aryl hydrocarbon receptor (AhR), activating transcription factor (ATF) 3, C/EBP homologous protein(CHOP), electrophile response element (EpRE), peroxisome preoliferator-activated receptor-gamma (PPAR-γ), and NF-E2-related factor (Nrf2).It has been shown that the nuclear factors, AP-1, NF-κB, STAT-3, β-catenin, Egr-1, HIF-1 Notch-1, are involved in cell proliferation, cell survival, invasion, angiogenesis, tumorigenesis and inflammation. In most cancers, these transcription factors are upregulated.
NF-κB , representing a family of eukaryotic transcription factors, plays anessential role in regulating the expression of a wide range of genes critical for innate and adaptive immunity, inflammation and cell survival. Dysregulated NF-κB activity occurs in a number of diseases, particularly cancer, chronic and acute inflammatory diseases. In non-stimulated cells, NF-κB is constitutively localized in the cytosol as a heterodimer by physical association with an inhibitory protein called inhibitor κB (IκB). Various pathogenic stimuli, including bacterial products, carcinogens, tumor promotors, cytokines, radiation, ischemia/reperfusion, and oxidants can activate NF-κB via several signal transduction pathways. Upon activation, NF-κB is translocated to the nucleus, where it induces the expression of more than 200 target genes that have been shown to induce cell proliferation, invasion, metastasis, chemoresistance, and/or inflammation.
The expression of constitutively active NF-κB has been reported in most of human cancer
cell line and tumors, including breast cancer, gynecologic cancer, gastrointestinal
cancer, head and neck squamous cell carcinoma, hematological cancer, and
melanoma. Curcumin prevents NF-κB activation induced by various agents through
inhibiting p65 translocation to the nucleus and suppressing IκBα degradation in numerous
cell types. By inhibiting NF-κB activation, curcumin suppresses the expression of
various cell survival and proliferative genes, including Bcl-2, Bcl-xL, cyclin D1, interleukin
(IL)-6, cyclooxygenase 2 (COX-2) and matrix metallopeptidase (MMP)-9, and subsequently
arrests cell cycle, inhibits proliferation, and induces apoptosis. (Aggarwal S, Takada Y, Singh S, et al. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor-kappaB signaling. Int J Cancer. 2004)
It has been identified that significant cross-talk and stimulation occurs between the Notch
and NF-κB pathways, both of which are important regulators of cell proliferation and survival, and are key factors in carcinogenesis. The Notch family of genes (Notch-1, -2,
-3, -4) encode a group of single-pass transmembrane cell-surface receptors that can be
activated by interacting with a family of its ligands. Upon activation, Notch is
cleaved, generating the nuclear transcriptional co-activator intracellular Notch (ICN).
Released ICN translocates into the nucleus where it functions as a transcriptional co-
activator by binding the CBF1/RBP-Jκ/Suppressor of Hairless/LAG-1 (CSL) transcription
factor. This binding event stimulates further co-activator recruitment and transcriptional
activation of Notch target genes that modulate key processes such as cell growth and
development, particularly hematopoietic events in cells of the immune system.
Curcumin downregulates Notch-1 signaling, which results in the inactivation of NF-κB
activity, and contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Furthermore, silencing expression of Notch-1 by RNA interference inhibits NF-κB
DNA binding and sensitizes cells to curcumin inhibition of cell growth, survival and NF-κB
activity. On the other hand, overexpression of Notch-1 attenuates curcumin-mediated cell
growth suppression, cell death, and NF-κB inhibition. AP-1, which was first known as a 12-O-tetradecanoylphorbyl-13-acetate (TPA) inducible transcription factor, is another transcription factor that regulates genes responsible for cell proliferation, survival, differentiation, apoptosis, cell migration, and transformation.(Wang Z, Zhang Y, Banerjee S, et al. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006)
AP-1 is a dimeric complex composed of many different proteins belonging to the c-Fos, c-
Jun, ATF and Jun dimerization protein families. These AP-1 factors can bind to the
TPA-response element sequence and enhance target gene expression.
The specific subunit composition of the AP-1 complex determines the diverse cellular responses to AP-1 activity(Dickinson DA, Iles KE, Zhang H, et al. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression)
Growth factors and protein kinases
Growth factors and their receptors play a critical role in the normal process of growth and
differentiation. Unregulated expression of these molecules can lead to abnormal growth and
development, resulting in malignant transformation. Curcumin has been shown to modulate the expression and activity of these growth factors, thereby exhibiting antiproliferative, anti-invasive an antiangiogenic effects. The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is an integral plasma membrane protein kinase that is composed of a cysteine-rich extracellular ligand- binding domain, a hydrophobic transmembrane domain, and intracellular C-terminal tails containing tyrosine kinase function and several tyrosine autophosphorylation sites [64,65]. It is a member of the ErbB family of receptors, which is a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her3 (ErbB-3) and Her4 (ErbB-4). The activation of EGFR occurs primarily through ligand-dependent mechanisms, but can also occur through ligand-independent events as well as through receptor overexpression. EGFR and its family members are stimulated by several distinct ligands, including epidermal growth factor (EGF), TGF-α, amphiregulin, betacellulin, epigen, epiregulin, and heparin binding EGF-like growth factor. Ligand binding to the extracellular domain of the receptor, induces the formation of receptor homo- or heterodimers. The formation of this receptor-dimer complex stimulates the auto- and/ or cross-phosphorylation of key tyrosine residues in the C-terminal tails of the receptor, which can function to initiate phosphorylation/signaling cascades via interaction with SH2- and phosphotyrosine-binding domain containing proteins. Dysregulated EGFR signaling has been implicated as a major contributing factor to many types of cancers, such as breast, lung, colorectal, and head/neck cancer. Specifically, the EGFR
pathway plays critical roles in cancer cell proliferation, migration, survival, angiogenesis,
and invasion.
The EGFR has been reported as a potential target of curcumin. Curcumin blocks
EGFR signaling by preventing EGFR tyrosine phosphorylation and suppressing EGFR gene
expression that is mediated by activation of PPAR-γ. Curcumin significantly inhibited
the proliferation and survival of lung adenocarcinoma PC-14 and pancreatic
adenocarcinoma p34 cells, which was associated with inhibition of phosphorylation of
extracellular receptor kinase (ERK) 1/2, and reduction of protein expression of COX-2 and
the EGFR. In addition, curcumin has been shown to inhibit the tyrosine kinase activity
of the HER2/neu receptor, and deplete the protein itself. Take together, suppression ofHER2/neu and EGFR activity represents one of the mechanisms by which curcumin suppresses the growth of breast cancer cells.(Levi-Ari S, Starr A, Vexler A, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk 1/2 activity)
The effects of curcumin are also apparently mediated through its inhibition of other protein
kinases, including autophosphorylation-activated protein kinase (AK), Ca2+-dependent
protein kinase (CDPK) , FAK , IL-1 receptor-associated kinase (IRAK), Janus
kinase (JAK), mitogen-activated protein kinases (MAPKs), the mammalian
target of rapamycin (mTOR), phosphorylase kinase (PhK), cytosolic protamine kinase (cPK), PKA, PKB/Akt, and spleen tyrosine kinase (Syk). Since these protein kinases are important for
cell growth, proliferation, survival, migration and other cellular events, inhibition of their
functions is undoubtedly one of the action mechanisms of curcumin. Below we will further
briefly discuss effects of curcumin on mTOR and MAPKs.
mTOR is a 289 kDa serine/threonine protein kinase that is a member of the PI3K-related kinase family of kinases . mTOR functions as a master regulator of numerous key cellular processes, including cell growth, proliferation, motility, survival, autophagy , protein synthesis, and RNA Polymerase I/II/III-mediated
transcription. mTOR also functions as a central sensor of cellular nutrient/amino
acid levels, cellular energy status, cellular redox status, and
mitogen stimulation, particularly from insulin, IGF-1, and IGF-2. Dysregulation
of the mTOR pathway is frequently observed in various human diseases, such as cancer and
diabetes. For example, activation of the mTOR pathway was noted in squamous cancers, adenocarcinomas, bronchioloalveolar carcinomas, colorectal cancers, astrocytomas and glioblastomas. These findings indicate a crucial role of mTOR signaling in tumorigenesis. mTOR functions as two distinct signaling complexes, mTOR complex 1/2 (mTORC1/2).(Johnson SM, Gulhati P, Arrieta I, et al. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling)
MAPKs are serine/threonine-specific protein kinases that play key roles in the transmission
of extracellular stimuli (mitogens or hormones) and the regulation of various cellular
activities, such as gene expression, mitosis, differentiation, cell proliferation, and cell
survival/apoptosis. Early studies showed that curcumin was able to inhibit c-jun N-
terminal kinase (JNK) activation induced by various agonists, including phorbol 12-
myristate 13-acetate (PMA) plus ionomycin, anisomycin, UV-C, gamma radiation, TNF-α,
and sodium orthovanadate. In MDA-MB-468 breast cancer cells, curcumin also
inhibited anisomycin-induced JNK activation, as well as EGF-induced phosphorylation of
ERK1/2. However, curcumin was able to induce cell apoptosis in cisplatin-resistant
ovarian cancer cells, in part by activation of p38 MAPK. In human astroglioma celllines, curcumin potently suppressed the phosphorylation of ERK, JNK, and p38 MAP
kinase, as well as MMP-9 enzymatic activity and protein expression. The study also
demonstrated that AP-1 was under the control of all three MAPKs, while NF-κB was
controlled by only two MAPKs, JNK and p38, suggesting that the inhibition of curcumin on
PMA-induced MMP-9 expression is mediated through the suppression of MAPKs and
subsequent inhibition of NF-κB and AP-1. The inhibitory effect of curcumin on
MAPKs pathway reveals a possible mechanism of AP-1 and NF-κB suppression, and these
effects may contribute to the potent anti-inflammatory and anti-cancer effects of this
chemical.
Inflammatory cytokines
During severe infection or after severe injury, excessive synthesis and production of pro-
inflammatory cytokines, including TNF-α, IL-1β and IL-6, play an major role in the
development of local and systemic inflammation, causing severe pathophysiological
derangement or organ failure. Cytokine gene and protein expression are tightly
controlled in the producing cells, and one of the most important steps in this regulation is
gene transcription. Therefore, inhibition of pro-inflammatory cytokine production by
regulation of transcriptional factors, such as NF-κB, is an potential strategy for controlling
inflammatory responses . Several studies have demonstrated that curcumin was
able to modulate the production of various inflammatory cytokines, thereby exhibiting
potent anti-inflammatory activity. For example in human multiple myeloma cells, curcumin inhibited IL-6-induced STAT3 phosphorylation and consequent STAT3 nuclear translocation, as well as the IFN-γ-induced STAT1 phosphorylation. Other inflammatory cytokines regulated by curcumin include CXCL1 and CXCL2, monocyte inflammatory protein-1 alpha (MIP-1α),monocyte chemoattractant protein-1 (MCP-1).
Adhesion molecules
Cell adhesion molecules (CAMs)are glycoproteins, which are located on the cell surface
and are required for binding with other cells or with the extracellular matrix in the process
called cell adhesion. Cell surface expression of various adhesion molecules, such as
intercellular cell adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1
(VCAM-1), and endothelial leukocyte adhesion molecule-1 (ELAM-1) plays a critical role
in inflammatory and neoplastic diseases. It has been reported that inhibition of
NF-κB completely blocked TNF-α-induced expression of ICAM-1, VCAM-1, and E-
selectin, indicating that the expression of CAMs is regulated in part by NF-κB [206]. In
human umbilical vein endothelial cells (HUVEC), curcumin potently inhibited TNF-α-
induced expression of ICAM-1, VCAM-1, and ELAM-1 partially through NF-κB inhibition,
and finally blocked HUVEC adhesion to monocytes. Most recent studies have also
shown that curcumin inhibited the expression of VCAM-1 in human intestinal microvascular
endothelial cells through suppression of Akt, p38 MAPK.
Integrins are a family of heterophilic CAMs that bind immunoglobulin superfamily CAMs or the extracellular matrix. During the last decade, studies on the function of integrins revealed that these molecules regulate an array of cellular processes, including cell death, proliferation, migration and differentiation. Most recent studies have
further shown that curcumin directly inhibited phosphorylation of β4 integrin (Y1494),
thereby inhibiting α6β4-mediated breast cancer cell motility and invasion.
Human clinical trials
Over the past years, a number of clinical trails have addressed the pharmacokinetics, safety
and efficacy of curcumin in humans. Consistent with the growing in vitro and in vivo
evidence of curcumin’s anti-inflammatory and anti-cancer properties, disease targets include
rheumatoid arthritis, postoperative inflammation, idiopathic inflammatory orbital pseudotumors, Alzheimer’s disease, multiple myeloma, pancreatic cancer, and colon cancer. The results from several preclinical and clinical trials showed that curcumin is remarkably well tolerated. Even at high doses, curcumin appears nontoxic to animals or humans. A Phase I clinical trial of curcumin was conducted in patients with high risk conditions or pre-malignant lesions of the bladder, skin, cervix, stomach or oral mucosa (Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions). When curcumin was administered as a single daily oral dose ranging from 500 to 8000 mg/day for 3 months, the treatment was well tolerated. In an independent dose-escalation study of curcuma extract on 15 patients with advanced colorectal cancer, curcumin extract was administered to patients at doses between 440 and 2200 mg/day, equivalent to 36-180 mg of curcumin, for up to 4 months. No treatment- related toxicity was observed at any doses either.
Although curcumin is well tolerated, and has a wide variety of beneficial activities, the in
vivo bioavailability of curcumin is poor, which may be an important obstacle to its utility as
a therapeutic agent. In a clinical study, it was shown that serum levels of curcumin
were undetectable or very low after 2 g of pure curcumin powder was administered alone to
fasting volunteers. A subsequent clinical phase I dose escalation study, which was
conducted in 15 patients with advanced colorectal cancer refractory to standard
chemotherapies, showed that consumption of 3.6 g of oral curcumin daily for up to 4 months
results in levels of drug and conjugates in plasma near the limit of detection of the assays
used, indicating the low systemic bioavailability of curcumin after oral dosing. This
finding is consistent with other data shown in preclinical models and in humans. Several pharmacokinetic studies of curcumin showed that curcumin seems to be metabolized through extensive conjugation and reduction in the gastrointestinal tract. Curcumin undergoes avid glucuronidation and sulfation leading to the formation of curcumin glucuronide and curcumin sulfates, as well as metabolic reduction to tetrahydrocurcumin and hexahydrocurcumin, which were found in intestinal and hepatic microsomes, and cytosol, respectively. Based on these results, it was suggested that the low systematic bioavailability of curcumin may be due to the hydrophobic nature of the molecule, poor absorption and the metabolic biotransformation in intestine and liver [Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far ].
Several approaches have been tried to improve the bioavailability of curcumin. These
approaches include: the use of adjuvants such as piperine to suppress glucuronidation in the
liver, the use of liposomal curcumin, curcumin nanoparticles, the use of curcumin phospholipid complex, and the use of structural analogs of curcumin.
In addition, curcumin has also been conjugated with other carriers, such as cyclodextrin and phosphatidylcholine. Shoba et al. reported that combined treatment with curcumin and piperine (20 mg/kg) produced higher serum concentration of curcumin from 0.25-1 h post-drug and increased the bioavailability by 2000% . A most recent study conducted to characterize the curcumin nanoparticles showed that the biodegradable
curcumin nanoparticulate formulation, which was based on poly (lactide-co-glycolide)
(PLGA) and a stabilizer polyethylene glycol (PEG)-5000, exhibited enhanced cellular
uptake, increased bioactivity in vitro, superior bioavailability in vivo and substantially longerhalf-life than curcumin. In another study, it was shown that curcumin-phospholipid
complex maintained effective concentration of curcumin for a longer time in rat serum and
proved better hepatoprotective activity than free curcumin at the same dose level.
However, there is still little information available about the in vivo efficacy and the safety of
those reformulated curcumin. Therefore, more studies are required to address these
questions.
Conclusions
For thousands of years, curcumin has been used in the Orient as a healing agent for a wide
range of inflammatory, neoplastic and other conditions. In recent years, the great therapeutic
potential against various human diseases, including cancer, cardiovascular diseases,
diabetes, arthritis, neurological diseases, and HIV-disease, has been documented. According
to the PubMed, over 3000 studies have been carried out with curcumin. This natural product
can modulate multiple cellular signaling pathways and affect numerous molecular targets.
Although curcumin is quite safe in humans, its low bioavailability may be a limitation for
clinical use. Various approaches are being undertaken to enhance the bioavailability of
curcumin. Obviously, more studies are needed to fully evaluate the efficacy and the safety of
reformulated curcumin, the structural analogues of curcumin as well as the combination of
curcumin with existing therapies. Nevertheless, the low cost, pharmacological safety, proven
therapeutic efficacy and multiple targeting potential make curcumin a promising agent for
prevention and treatment of various human diseases. Meanwhile, reformulations of
curcumin with enhanced bioavailability may also hold great promise in the future.
Curcumin attenuates urinary excretion of albumin in type II diabetic patients with enhancing nuclear factor erythroid-derived 2-like 2 (Nrf2) system and repressing inflammatory signaling efficacies. 2015
In addition, curcumin reduced plasma MDA level with enhanced the Nrf2 system specifically regulated protein, NADH quinone oxidoreductase 1 (NQO-1) together with other anti-oxidative enzymes in patients' blood lymphocytes.