
Description

Fingolimod (also called with his trade name Gilenya or with the name code FTY720) is an immunomodulating drug, who has been recently approved as treatment of multiple sclerosis. Fingolimod is the first oral tratment for Multiple sclerosis (MS) and is the first to show a double action: the first action is towards the linfatic system, sequestering lymphocytes in lymph nodes and preventing them from moving to the central nervous system for autoimmune responses; the seconde innovative aspect is the direct influence on the cells of central nervous sistem, stimulating the repair process of glial cells and precursor cells after injury.
Fingolimod is derived from the myriocin (ISP-1), metabolite of the fungus Isaria sinclairii.
It is a structural and functional analogue of the natural serum lipid, sphingosine, and gets phosphorylated by sphingosine kinases in the cell (sphingosine kinase 1 predominating in the lungs and spleen and sphingosine kinase 2 predominating in the heart, brain and liver).
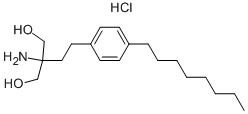
The chemical name for fingolimod is 2-amino-2-[2-(4-octylphenyl)ethyl]propan-1,3-diol hydrochloride.
Classification
Immunosuppressive drug
Indications
Fingolimod has been invented as immunosuppressive treatment in kidney transplantation; but in a previous phase III trial of kidney transplantation fingolimod was found to be no better than the existing standard of care.
At the moment fingolimod is indicated only for the treatment of patients with relapsing-remitted (RR) forms of multiple sclerosis (MS) to reduce the frequency of clinical exacerbations and to delay the accumulation of physical disability.
Studies currently underway to determine its potential benefit for persons with primary-progressive MS; and fingolimod has recently been discovered as a candidate therapeutic drug for the treatment of arrhythmias and hypertrophic/fibrotic heart disease .
Pharmacokinetics
Absorption
After oral administration of one dose, blood concentration of fingolimod increase for 12 hours; The Cmax of fingolimod is 12-16 hours. Pharmacokinetic profile in human patients shows a linear, dose-dependent relationship for the maximum concentration and the area under the curve. The apparent absolute oral bioavailability is 93%. Food intake does not alter Cmax or exposure (AUC) of fingolimod or fingolimod-phosphate (fingolimod may be taken without regard to meals).
Fingolimod has a half-life of 6 to 9 days, and steady-state blood concentrations are reached within 1 to 2 months following once-daily administration (steady-state levels are approximately 10-fold greater than with the initial dose).
Distribution
Local concentrations of both phosphorylated and unphosphorylated fingolimod are much higher in lymphoid tissues than in blood, generating a reservoir in thymus and secondary lymphoid organs, which leads to sustained fingolimod production and activation of S1P within tissues. Fingolimod highly (86%) distributes in red blood cells, while fingolimod-phosphate has a smaller uptake in blood cells of < 17%. Fingolimod and fingolimod-phosphate are > 99.7% protein bound and fingolimod and fingolimod-P protein binding is not altered by renal or hepatic impairment. Fingolimod is extensively distributed to body tissues with a volume of distribution of about 1200±260 L.
Metabolism
There are three main pathways, in humans, for the biotransformation of fingolimod:
- by reversible stereoselective phosphorilylation to the pharmacologically active (S)-enantiomer of fingolimod-phosphate,
- by oxidative biotransformation mainly via the cytochrome P450 4F2 isoenzyme and subsequent fatty acid-like degradation to inactive metabolites,
- by formation of pharmacologically inactive non-polar ceramide analogs of fingolimod.
Fingolimod is primarily metabolized via human CYP4F2 (with a minor contribution of CYP2D6, 4F12, 3A4, and 2E1); inhibitors or inducers of these isozymes might change the exposure of fingolimod or fingolimod-P. The involvement of multiple CYP isoenzymes in the oxidation of fingolimod suggests that the metabolism of fingolimod will not be subject to substantial inhibition in the presence of an inhibitor of a single specific CYP isozyme.
Pharmacokinetic interactions of other substances on fingolimod
Fingolimod is metabolised mainly by CYP4F2 (80%), but other enzymes like CYP3A4 may also contribute to its metabolism. Co-administration of fingolimod with substance that may inhibit CYP4F2 or CYP3A4 should be done with caution, because it could leed to increase in fingolimod and fingolpimod-P exposure (AUC). These substance are for example: Ketoconazole, protease inhibitors, some macrolides as clarithromycin or telithromycin, azole antifungals.
Elimination
Fingolimod blood clearance is 6.3±2.3 L/h, and the half-life (t½) is 6-9 days. Blood levels of fingolimod-phosphate decline in parallel with those of fingolimod in the terminal phase.
After oral administration, about 81% of the dose is slowly excreted in the urine as inactive metabolites. Fingolimod and fingolimod-phosphate are not excreted intact in urine but are the major components in the feces with amounts of each representing less than 2.5% of the dose.
Pharmacodynamics and Molecular mechanism
Fingolimod is a sphingosine1-phosphate receptor (S1P receptor) modulator.
Fingolimod is pro-drug, so it need to get phosphorylated by sphingosine kinases.
Afeter phosphorylation, as structural analogue of the sphingosine it’s able to bind S1P receptor and to activate segnalling pathway.
Initially fingolimod-P acts as an S1P-receptor agonist; however, chronic exposure to fingolimod-P leads to irreversible receptor internalization resulting in ‘functional antagonism’ of S1P1-mediated S1P signaling. In fact acute agonism of S1P-receptor with FTY720-P leads to similar cellular effect as S1P, including intracellular calcium rise, mitogen-activated protein kinase activation, adenylate cyclase inhinition, vascular endothelial-cadherin assembly, and cell migration.
Both S1P and Fingolimod induce receptor internalization into the endosomal pathway. However, in fingolimod treated cells the S1P-receptor does not recycle to the cell membrane.
The mechanisms involved are poorly understood. A work of 2007 suggested that the cause of this down-regulation may be linked to the ubiquitinylation and proteasomal degradation pathway, the study shows the exaggerated ubiquitinylation induced by fingolimod-P targets the receptor to the proteasomal degradative pathway; the higher affinity of fingolimod (compare to S1P) to S1P-receptors leads to tight binding and less recycling of S1P receptors and, as a consequence, to proteasomal degradation of the drug-receptor complex.
Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor

Biochemical and physiological effects on the body
S1P is produced by phosphorylation of sphingosine by ubiquitously expressed sphingosine kinases. There are five known S1P receptor subtypes, S1P1–5, which belong to the G protein-coupled receptor super family and are expressed on a lot of cell types (such as lymphocytes and neural cells).
- S1P1–3 are widely distributed in the immune and cardiovascular systems and the Central Nervous Sistem (CNS).
- S1P1 is highly expressed on T and B lymphocytes.
- S1P4 is generally confined to lymphoid and hematopoietic tissues.
- S1P5 is predominantly located in the CNS white matter.
So S1P receptors are involved in a wide range of biological processes: leukocyte recirculation, neural cell proliferation, migration, morphological changes, vasoregulation, endothelial cell function and cardiovascular development.
Role of S1P receptor and effecst of fingolimod in the immune system:

S1P and his receptor are necessary for the egress of thymocytes from the thymus, and for the egress of T and B cells from lymphoid tissue. In fact the retention in lymphoid tissue and recirculation of lymphocytes to the blood circulation is regulated by the concentration gradient of S1P between lymphoid tissue and other tissues, sensed through S1P1 on lymphocytes. After clonal expansion of the activated T cells, cell expression of S1P1 on the surface is upregulated, allowing to the cells to respond to the gradient which drives egress of lymphocyte into the circulation. S1P interaction with his receptor let to overcome the retention signals mediated by chemokine receptor 7 (CCR7) expressed on B cells and naive and central memory T cells.
On phosphorylation, Fingolimod initially activates lymphocyte S1P1 via high-affinity receptor binding, yet later induces S1P1 down-regulation (the receptor are internalized and degraded, reducing or eliminating them from the lymphocyte cell surface) that prevents lymphocyte egress from lymphoid tissues and prevents these cell from infiltrating inflammatory lesion into the central nervous system (CNS). This downregulation renders lymphocytes unresponsive to the normal S1P gradient and thus deprives them of the obligatory signal that allow them to egress from lymphoid tissues and recirculate to the periphery. Recent studies show that fingolimod prevents the egress of CCR7-positive naive T cells and central memory cells from lymph nodes, but to spare CCR7-negative effector memory cells.
An other activity of fingolimod that has been shown is the reduction of the number of Th17 cells in peripheal blood; pro-inflammatory Th17 cells produce the inflammatory cytokine interleukin 17 (IL-17) and they have been implicated in MS pathogenesis.
Accumulating data indicate that while fingolimod modulates lymphocyte egress, it does not inhibit lymphocyte effector functions; many normal immune response functions are therefore maintained during treatment. Lymphopenia produced by fingolimod differs from that caused by immunosuppressants such as azathioprine, which inhibit lymphocytes from forming, whereas fingolimod simply sequesters lymphocytes in the lymph nodes.
Role of S1P and effect of fingolimod on CNS cell:
The innovative action of fingolimod is the direct effects within the central nervous sistem. Fingolimod crosses the blood–brain barrier and may therefore have direct CNS effects, distinguishing it from immunologically targeted MS therapies.
S1P and receptors have an influence on cell proliferation, neural cell function, morphology and migration. By modulating the S1P receptors expressed on CNS cells, fingolimod may have a direct impact on neuropathological processes such as neurodegeneration, gliosis and endogenous repair mechanisms.
S1P signalling has effect on oligodendrocytes in remyelination process, that require cellulare processes of proliferation, migration, adhesion, process extension/retraction and differentiation. Fingolimod exposure can cause the increase of number of both progenitor and mature oligodendrocyte, can protect oligodendrocytes from cell death induced by cytokines or the withdrawal of growth factors, and it can modulate process outgrowth. S1P promote the migration of neural stem/progenitor cell towars areas of damage in the CNS. Fingolimod increased the levels of the endogenous neuroprotectant, brain-derived neurotrophic factor in a dose-, time- and activation dependent manner. Fingolimod also influences S1P receptor-mediated signaling and migration of the astrocytes; it also change the composition of the blood-brain barrier, inducing adherens junction assembly and reducing vascular leakage (attenuating ceramide-induced blood-brain barrier dysfunction in multiple sclerosis by targeting reactive astrocytes, which are the primary cellular source of enhanced ceramide production).
Pharmacogenomics
Prevalence of functionally relevant single-nucleotide polymorphisms in genes involved in fingolimod metabolism in patients with multiple sclerosis: a Gilenya-pharmacogenomic pilot study
Drug interactions
- Fingolimod may interact with antiarrhythmics (eg, amiodarone, disopyramide, dofetilide, dronedarone, ibutilide, procainamide, quinidine, sotalol), beta-blockers (eg, atenolol, metoprolol), beta-2 receptor agonists (eg, salmeterol), calcium channel blockers (eg, diltiazem, verapamil), chloroquine, chlorpromazine, cholinesterase inhibitors (eg, galantamine), citalopram, digoxin, domperidone, fluoxetine, haloperidol, macrolide antibiotics (eg, erythromycin), methadone, ondansetron, pilocarpine, quinine, quinolone antibiotics (eg, ciprofloxacin), tacrolimus, tetracyclic antidepressants (eg, maprotiline), tricyclic antidepressants (eg, amitriptyline), tyrosine kinase inhibitors (eg, sunitinib), or vorinostat because the risk of slow or irregular heartbeat may be increased; indeed these drugs work as pharmacodynamic synergist and bring to increased risk of bradycardia, AV block, and torsade de pointes.
- Antineoplastic, corticosteroids (eg, prednisone), drug with effect on immune system, immunosuppressive (like belimumab) or immune modulating therapies used for treatment of MS (eg, interferon beta, mitoxantrone, natalizumab) because the risk of infection may be increased. Vaccination may be less effective during and for up to 2 months after discontinuation of treatment Fingolimod and the use of live attenuated vaccines should be avoided during and for two months after treatment with fingolimod because of the risk of infections.
- Azole antifungals (eg, ketoconazole), macrolide antibiotics (eg, erythromycin), protease inhibitors (eg, ritonavir), or telithromycin because they may increase the risk of fingolimod's side effects.
Side effects
In general, the most frequently reported side effects (incidence >10%) have included headache, influenza, diarrhea, back pain, increased liver enzymes, and cough; the increased liver enzymes were the only adverse effect which lead to treatment interruption (3.8%).
There are other specific short-range and long-range side effects:
Cardiovascular side effects have included:
- Bradycardia (4%). Tipically the first dose of fingolimod results in a bradycardia that peaks 6 hours after dosing; the bradycardia rarely (0.5%) results in symptoms. It is recommended that the patient be observed for the first six hours after the first dose of fingolimod and treated if symptomatic bradycardia occurs.
- Hypertension(6%) and vascular events. In clinical trials with fingolimod, a small sustained increase in blood pressure was seen after 2 months of continued therapy. The increase averaged approximately 2mm Hg systolic and 1mm Hg diastolic blood pressure. Hypertension was reported as an adverse reaction in 5% of patients on fingolimod 0.5mg and 3% on placebo.
- Vascular events including ischemic and hemorrhagic strokes, peripheral arterial occlusive disease, and posterior reversible encephalopathy syndrome (PRES) have been reported in clinical trials with higher doses of fingolimod, but were not observed with the 0.5mg dose
Hepatic side effects have included increased aspartate/alanine (AST/ALT) aminotransferases (14%) and increased gamma glutamyl transpeptidase (GGT; 5%). Most elevations occurred at 3 to 4 months of treatment and returned to baseline if treatment was discontinued. It is recommended that transaminase and bilirubin determinations be obtained prior to starting treatment and repeated if symptoms suggestive of hepatic dysfunction occur. Patients with preexisting liver disease may be at increased risk of hepatotoxicity with fingolimod.
Immunologic side effects have included influenza viral infection (13%), herpes viral infection (9%), and tinea infections (4%).
While the overall rate of infections (72%) and serious infections (2%) on the recommended (0.5 mg) dosing of fingolimod was similar to placebo, the rates of bronchitis and pneumonia were somewhat higher in the fingolimod group – and two patients on the higher dose of fingolimod (1.25 mg daily) died of herpetic infections, one of whom lacked immunity to varicella-zoster virus (VZV). It is recommended that fingolimod not be started in patients with active acute or chronic infections.
Ocular side effects have included blurred vision (4%), eye pain (3%), and macular edema.
Because macular edema occurred in 0.4% of treated patients, it is recommended that patients undergo ophthalmologic evaluation at baseline and at 3 to 4 months of treatment. The risks of macular edema in patients with a history of uveitis or diabetes mellitus appear higher, and periodic monitoring for macular edema is recommended in such patients.
Respiratory side effects have included cough (10%), dyspnea (8%), bronchitis (8%), sinusitis (7%) and decrease in pulmonary function; dose dependent reductions in FEV1 and carbon monoxide diffusion capacities were observed in patients on fingolimod. These reductions increased with duration of therapy. The changes were rarely symptomatic – with only a few patients discontinuing therapy due to dyspnea – and the changes in FEV1 appeared to be reversible upon discontinuation of therapy.
Dermatologic side effects have included alopecia (4%), eczema (3%), and pruritus (3%).
Gastrointestinal side effects have included diarrhea (12%) and gastroenteritis (5%).
Hematologic side effects have included lymphopenia (4%) and leucopenia (3%).
Metabolic side effects have included weight loss (5%) and increased serum triglycerides (3%).
Musculoskeletal side effects have included back pain (12%).
Nervous system side effects have included headache (25%), dizziness (7%), paresthesia (5%), and migraine (5%).
Psychiatric side effects have included depression (8%).
Other side effects have included asthenia (3%) and fetal death and malformations. Animal studies suggest that fingolimod may cause fetal harm, including embryo-fetal death and fetal malformations; women who wish to become pregnant should be counseled that it takes approximately 2 months to clear fingolimod after discontinuing treatment, so they should avoid becoming pregnant within that period.
Contraindications
The principle contraindications are for patients who in the last 6 months experienced myocardial infarction, stroke, severe sleep apnea, unstable angina, TIA, decompensated heart failure requiring hospitalization or Class III/IV heart failure.
Also history or presence of Mobitz Type II second-degree or third-degree atrioventricular (AV) block or sick sinus syndrome (unless patient has a functioning pacemaker) represents a contraindication for treatment with Fingolimod.
This is because of the fingolimod’s action that induces bradycardia.
Other controindications are represent by: known immunodeficiency syndrome; patients with increased risk for opportunistic infections, including immunocompromised patients (including those currently receiving immunosuppressive therapies or those immunocompromised by prior therapies); Severe active infections and active chronic infections (hepatitis, tuberculosis); known active malignancies, except for patients with cutaneous basal cell carcinoma and severe liver impairment (Child-Pugh class C).
Overdose
Possible effects of an overdose with Gilenya include but are not limited to:
- Chest tightness or discomfort.
- A slow heart rate (bradycardia)
An overdose with this medication may also cause typical fingolimod side effects.
Deliberate Fingolimod Overdose Presenting with Delayed Hypotension and Bradycardia Responsive to Atropine